Limertinib: A Novel Third-Generation EGFR-TKI Approved in China
Limertinib is a newly developed small-molecule chemical drug, specifically designed as an inhibitor targeting the epidermal growth factor receptor (EGFR) T790M mutation. It received market approval from the National Medical Products Administration (NMPA) on January 16, 2025.
Mechanism of Action
The epidermal growth factor receptor (EGFR) is a transmembrane protein belonging to the ErbB receptor family. It plays a critical role in regulating cell proliferation, differentiation, and survival. Mutations in EGFR, particularly those that result in abnormal activation of its tyrosine kinase activity, can lead to uncontrolled cell growth, a hallmark of many cancers, especially non-small cell lung cancer (NSCLC).
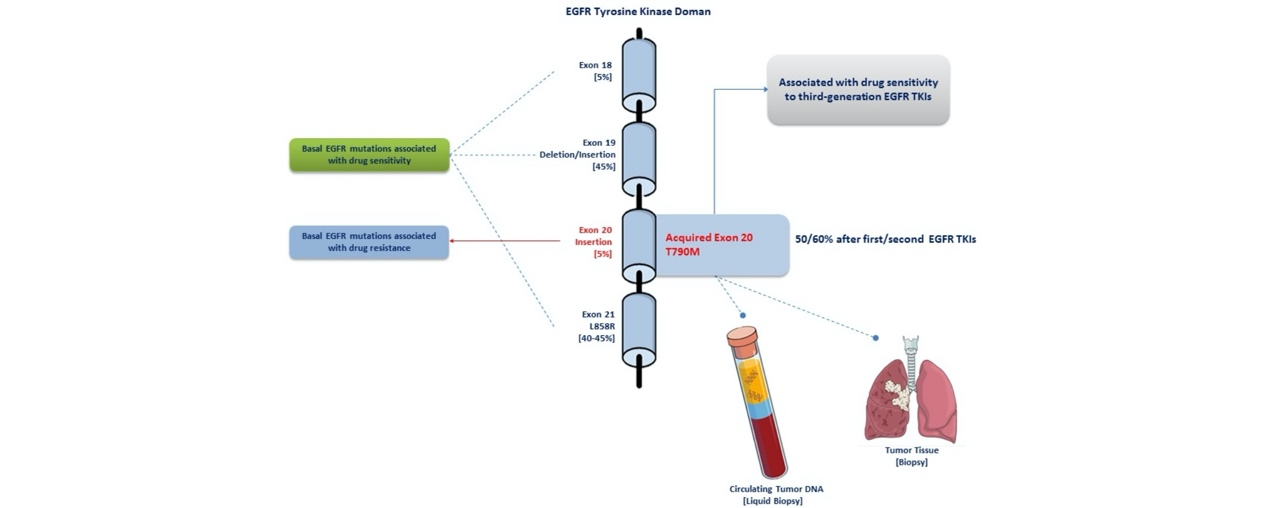
The EGFR T790M mutation refers to a substitution of threonine (T) with methionine (M) at codon 790 in exon 20 of the EGFR gene. This mutation typically arises in patients who have been previously treated with first- or second-generation EGFR tyrosine kinase inhibitors (TKIs) and is a major mechanism of acquired resistance to these therapies.
The T790M mutation increases the affinity of EGFR for ATP, thereby reducing the ability of first- and second-generation EGFR-TKIs to bind effectively to the receptor. These earlier-generation inhibitors function primarily by competitively inhibiting ATP binding to block EGFR signaling. However, third-generation EGFR-TKIs, such as Osimertinib, Almonertinib, and the newly approved Limertinib, are designed to specifically target the EGFR T790M mutation, effectively overcoming resistance caused by this mutation.
As a novel third-generation EGFR-TKI, Limertinib has demonstrated promising efficacy in clinical trials. It is effective not only in adult patients with locally advanced or metastatic NSCLC harboring the EGFR T790M mutation but also in patients with central nervous system (CNS) metastases. This suggests that Limertinib not only effectively inhibits tumor growth but also has the ability to penetrate the blood-brain barrier, providing a new treatment option for patients with brain metastases.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Reference:
- 1. Passaro A, Guerini-Rocco E, Pochesci A, Vacirca D, Spitaleri G, Catania CM, Rappa A, Barberis M, de Marinis F. Targeting EGFR T790M mutation in NSCLC: From biology to evaluation and treatment. Pharmacol Res. 2017 Mar;117:406-415. doi: 10.1016/j.phrs.2017.01.003. Epub 2017 Jan 12. PMID: 28089942.