Longbio Pharma Presents Promising LP-003 Drug Data at AAD 2024
Longbio Pharma Co., Ltd., an eminent enterprise in the biotechnological field devoted to the creation of novel protein-based therapies aimed at allergy, lung conditions, skin disorders, blood-related illnesses, eye health, as well as various autoimmune and uncommon diseases, enthusiastically unveiled the initial stage clinical trial results for its pioneering treatment, LP-003, during the yearly conference of the American Academy of Dermatology Association held in 2024.
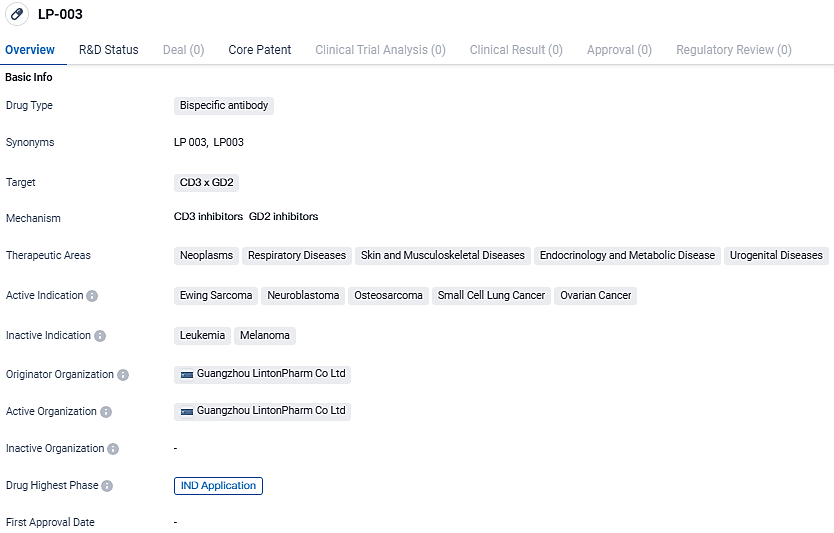
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
During the symposium, Longbio Pharma's unveiling of their poster, entitled “A Phase 1 Study in Healthy Participants Examining LP-003, an Innovative Extended-Duration, High Specificity Anti-IgE Monoclonal Antibody,” signified an important event.
This initial Phase I clinical trial was designed as a randomized, placebo-controlled, single-dose escalation study involving 32 volunteers. These individuals were split into five distinct cohorts to receive one intravenous infusion of 0.3 mg/kg, 1 mg/kg, 3 mg/kg, 6 mg/kg, or 10 mg/kg of the drug. Investigators monitored for safety, as well as assessed pharmacokinetic and pharmacodynamic responses.
The pharmacokinetic analysis indicated an anomalous pattern, with LP-003's elimination half-life varying between 44.6 and 76.5 days, nearly double to triple the duration seen with Omalizumab. Notably, concentrations of free-IgE plunged below measurable limits for a span exceeding 168 days following administration within the 1 mg/kg to 10 mg/kg dosage range.
The tolerance profile for LP-003 was favorably received, with no incidents of severe treatment-emergent adverse events (Grade 3 or higher) observed, providing a robust platform for future research and progressive development steps.
Findings from the subsequent Phase II investigation of Ligelizumab suggest that anti-IgE monoclonal antibodies with enhanced affinity for IgE and intensified therapeutic action may prove to be more efficacious than Omalizumab.
Throughout this study, Longbio Pharma has pioneered a next-gen anti-IgE monoclonal antibody, LP-003, demonstrating superior IgE binding affinity and potentiated FcεRI inhibition activity, which surpasses both Omalizumab and Ligelizumab. Importantly, LP-003 also achieves a markedly prolonged half-life in relation to Omalizumab.
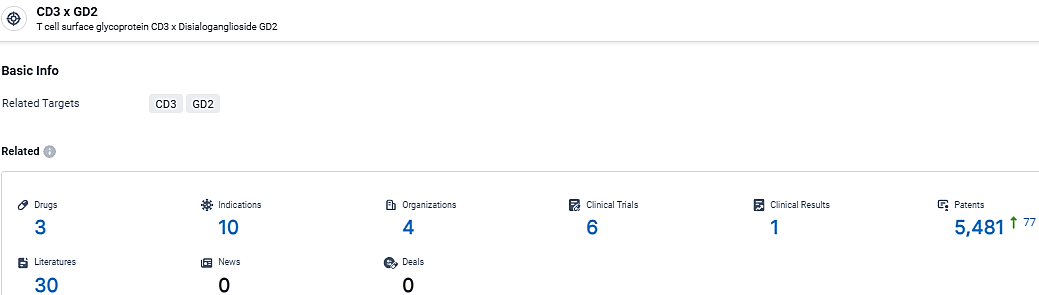
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 14, 2024, there are 3 investigational drugs for the CD3 and GD2 target, including 10 indications, 4 R&D institutions involved, with related clinical trials reaching 6, and as many as 5481 patents.
Bispecific antibodies like LP-003 have gained significant attention in the pharmaceutical industry due to their ability to simultaneously target two different antigens. By targeting CD3 and GD2, LP-003 aims to enhance the immune response against cancer cells, potentially leading to improved treatment outcomes for patients with the indicated diseases.