Marinus Pharmaceuticals Announces Progress in Phase 3 RAISE Study and Delivers Early Financial Figures for Q1 2024
Marinus Pharmaceuticals, Inc., a biopharmaceutical company committed to creating novel treatments for seizure-related conditions, has declared that the independent Data Monitoring Committee has advised proceeding with the crucial Phase 3 RAISE trial. This study assesses intravenous ganaxolone in treating refractory status epilepticus, based on the outcomes of an interim analysis.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Marinus has announced plans to conclude patient enrollment for the RAISE trial at around 100 participants, with preliminary results anticipated by summer 2024. These findings will help ascertain whether further development of IV ganaxolone should proceed. Currently, Marinus does not have access to the RAISE trial's data.
"Although it's unfortunate that the RAISE trial didn't achieve the early termination conditions, a clear verdict on the trial can only be shaped after we process the complete set of unblinded data," mentioned Scott Braunstein, M.D., Chairman and CEO of Marinus. He added, "We are also looking into methods to lower costs to maintain a robust financial status as we near the end of enrolling participants in the global Phase 3 TrustTSC trial targeting tuberous sclerosis complex."
Enrollment for the Phase 3 TrustTSC trial involving ZTALMY(ganaxolone) oral suspension CV is expected to wind up around mid-May 2024 with roughly 130 patients. Marinus forecasts the main results by early fourth quarter of 2024 and plans to submit a supplemental New Drug Application to the FDA in early 2025, hoping to secure a priority review.
Alongside, Marinus is progressing with the development of an advanced ganaxolone formula that aims to enhance pharmacodynamic and pharmacokinetic attributes, potentially increasing safety, efficacy, and tolerability, while also reducing the frequency of dosages.
ZTALMY's commercial release in the U.S. has been successful, bringing in estimated unaudited net product revenues of $7.4 to $7.6 million for the first quarter of 2024. As of March 31, 2024, Marinus estimates its unaudited cash on hand and short-term investments to be $113.3 million. The company is considering cost-cutting measures to lengthen its financial runway past Q4 2024, with implementations expected soon.
Please note, the financial figures for Q1 2024 including net product revenues, cash, and investments are preliminary, calculated before review by the independent registered public accounting firm of the company and might be subject to modifications.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
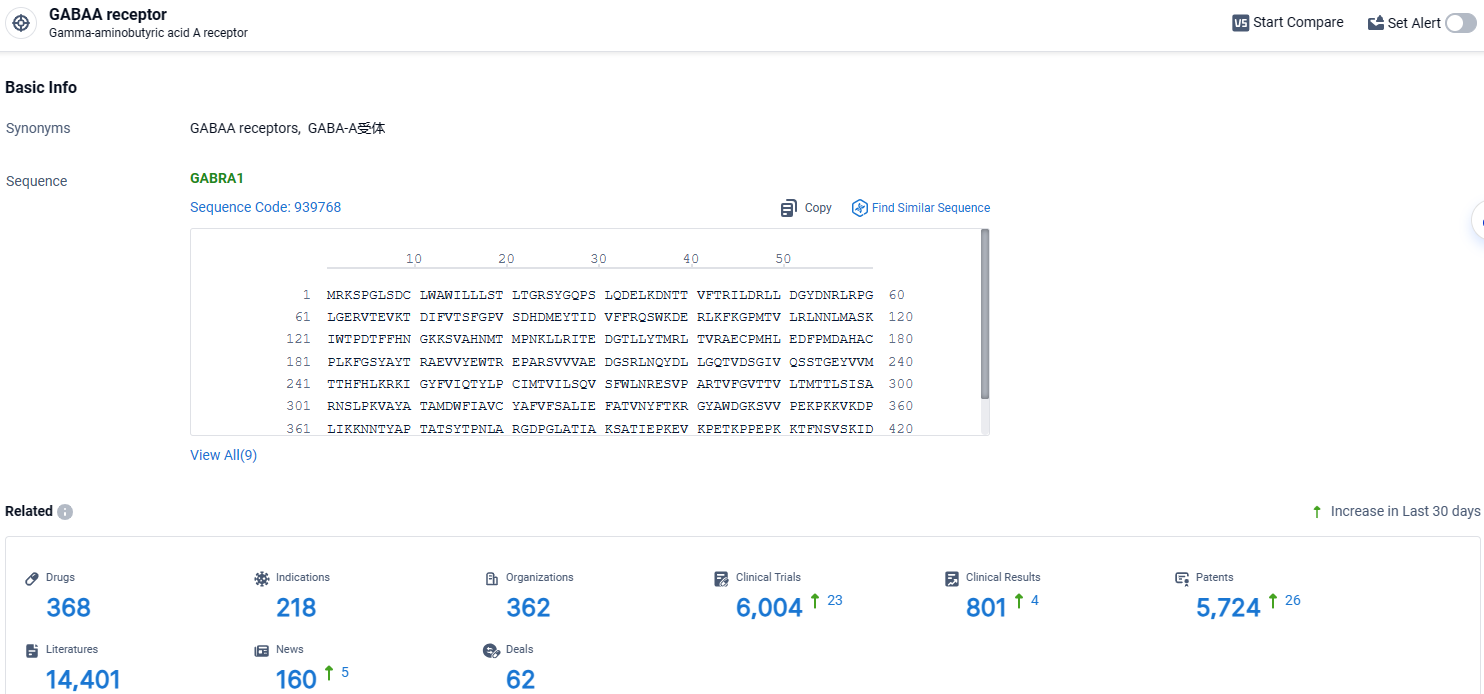
According to the data provided by the Synapse Database, As of April 17, 2024, there are 368 investigational drugs for the GABAA receptor target, including 218 indications, 362 R&D institutions involved, with related clinical trials reaching 6004, and as many as 5724 patents.
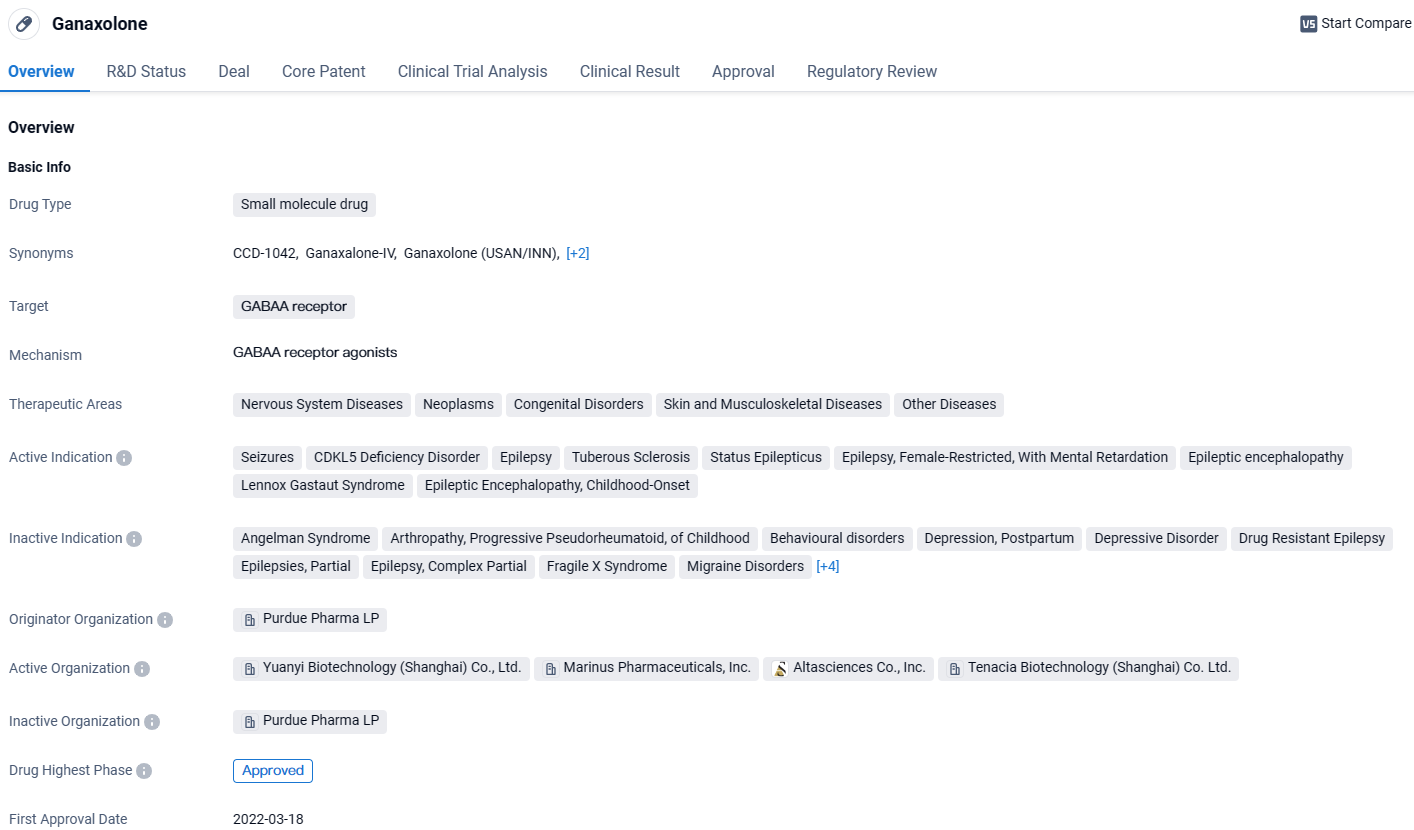
Ganaxolone is a small molecule drug that targets the GABAA receptor and is used in the treatment of various nervous system diseases, neoplasms, congenital disorders, skin and musculoskeletal diseases, and other diseases. It has received approval globally and is currently seeking approval in China. The drug has shown efficacy in treating a range of conditions, including seizures, epilepsy, and epileptic encephalopathy. Its first approval was granted in the United States in 2022, and it is regulated under priority review, rare pediatric disease, and orphan drug designations.