MK-4830: A Quick Look at Its R&D Progress and Clinical Results from the 2024 AACR
ILT4 is an immunosuppressive receptor commonly expressed on myeloid cells. Antagonism of ILT4 may induce a proinflammatory state and stimulate antitumor T-cell response, especially with PD-(L)1 blockade. On April 7, 2024, the latest clinical data of the anti-ILT4 IgG4 monoclonal antibody, MK-4830, in combination with pembrolizumab (pembro) in patients with previously untreated advanced head and neck squamous cell carcinoma (HNSCC) or non-small cell lung cancer (NSCLC) were reported in 2024 AACR.
MK-4830's R&D Progress
The drug MK-4830 is a monoclonal antibody that targets LILRB2. Monoclonal antibodies are a type of drug that are designed to bind to specific proteins in the body and can be used for various therapeutic purposes. LILRB2 is the specific target of MK-4830, and it is involved in various diseases and conditions. In terms of therapeutic areas, MK-4830 is being developed for the treatment of neoplasms, endocrinology and metabolic diseases, urogenital diseases, and digestive system disorders.
According to the Patsnap Synapse, MK-4830 has reached the highest phase of Phase 2 globally. And the clinical trial distributions for MK-4830 are primarily in the United States, China and United Kingdom. The key indication is Esophageal Squamous Cell Carcinoma.
Detailed Clinical Result of MK-4830
This non-randomized, parallel assignment, open-labeled study (NCT03564691) was aimed to evacuate the safety, efficacy, and biomarker analyses for MK-4830 + pembro from the multi-cohort expansion phase of patients (pts) with previously untreated advanced HNSCC or NSCLC.
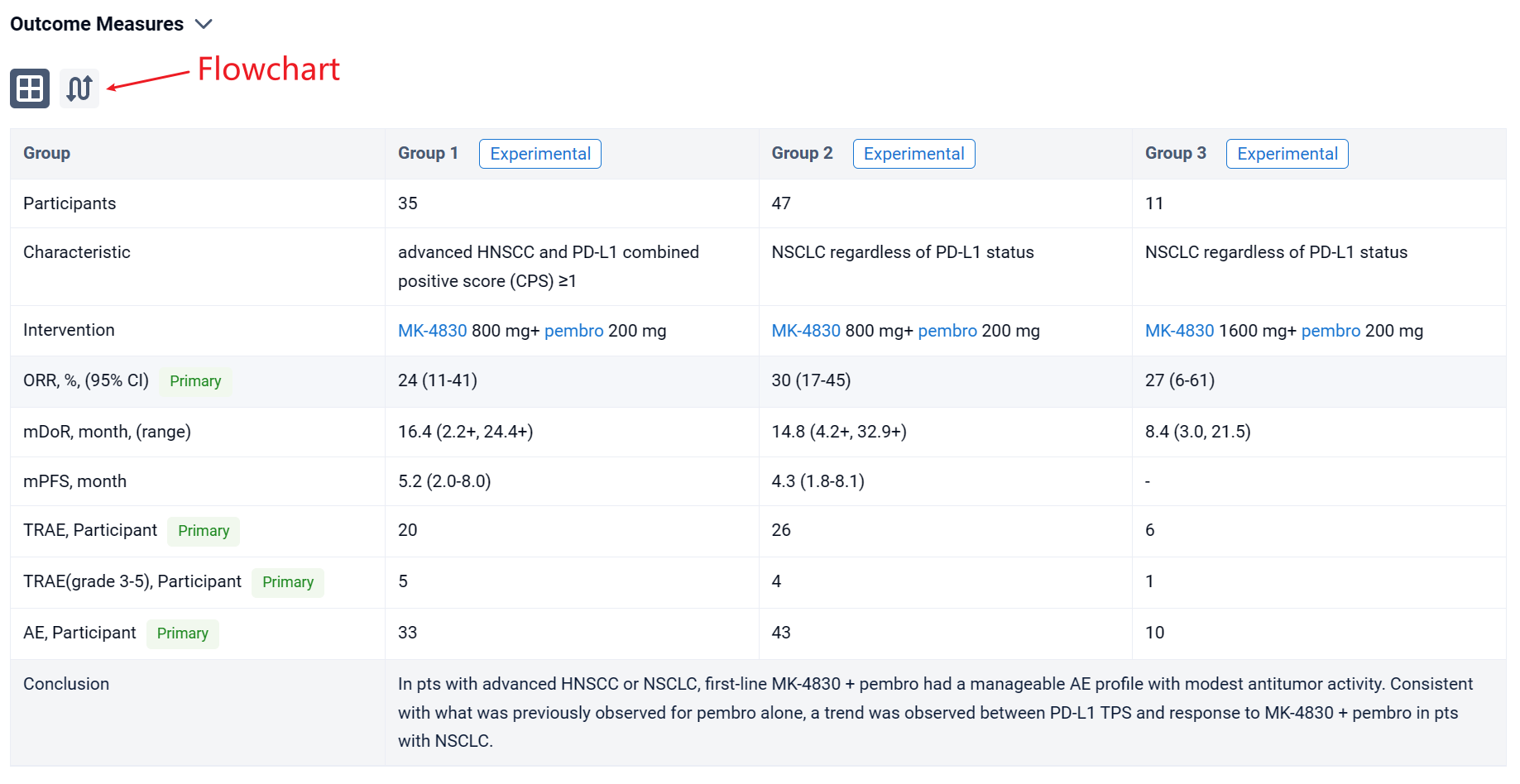
In this study, pts aged ≥18 y with previously untreated advanced HNSCC and PD-L1 combined positive score (CPS) ≥1 (cohort D) or NSCLC regardless of PD-L1 status (cohorts E and F) were enrolled. Pts in all cohorts received either MK-4830 800 mg (cohorts D and E) or 1600 mg (cohort F) + pembro 200 mg Q3W for ≤35 cycles. Primary end points were safety and ORR per RECIST v1.1 by investigator assessment. Exploratory end points included DOR and PFS per RECIST v1.1 by investigator assessment and evaluating possible biomarkers of response to MK-4830 + pembro.

The result showed that at data cut-off (September 27, 2023), 35, 47, and 11 pts were enrolled and received treatment in cohorts D, E, and F, respectively. Median study follow-up was 34.4 mo (range, 26.5-42.4) in cohort D, 35.9 mo (22.4-39.8) in cohort E, and 38.7 mo (37.6-39.6) in cohort F. Adverse events (AEs) occurred in 33 pts (94%) in cohort D, 43 pts (91%) in cohort E, and 10 pts (91%) in cohort F. Treatment-related AEs (TRAEs) occurred in 20 pts (57%) in cohort D, 26 pts (55%) in cohort E, and 6 pts (55%) in cohort F; grade 3-5 TRAEs occurred in 5 pts (14%), 4 pts (9%), and 1 pt (9%), respectively. One pt in cohort E and 1 pt in cohort F died due to a TRAE (pneumonitis and pneumonia, respectively). ORR was 24% (95% CI, 11-41) in cohort D, 30% (17-45) in cohort E, and 27% (6-61) in cohort F. Median DOR was 16.4 mo (range, 2.2+ to 24.4+) in cohort D, 14.8 mo (4.2+ to 32.9+) in cohort E, and 8.4 mo (3.0-21.5) in cohort F. Median PFS was 5.2 mo (95% CI, 2.0-8.0) in cohort D, 4.3 mo (1.8-8.1) in cohort E, and 3.3 (1.2-7.6) in cohort F. In cohort D, T-cell-inflamed gene expression profile (TcellinfGEP) and tumor mutational burden (TMB) did not enrich for response to MK-4830 + pembro. In cohorts E and F, PD-L1 tumor proportion score (TPS) and TcellinfGEP trended higher in pts with response to MK-4830 + pembro than pts with no response; TMB did not trend with response.
It can be concluded that in pts with advanced HNSCC or NSCLC, first-line MK-4830 + pembro had a manageable AE profile with modest antitumor activity. Consistent with what was previously observed for pembro alone, a trend was observed between PD-L1 TPS and response to MK-4830 + pembro in pts with NSCLC.
How to Easily View the Clinical Results Using Synapse Database?
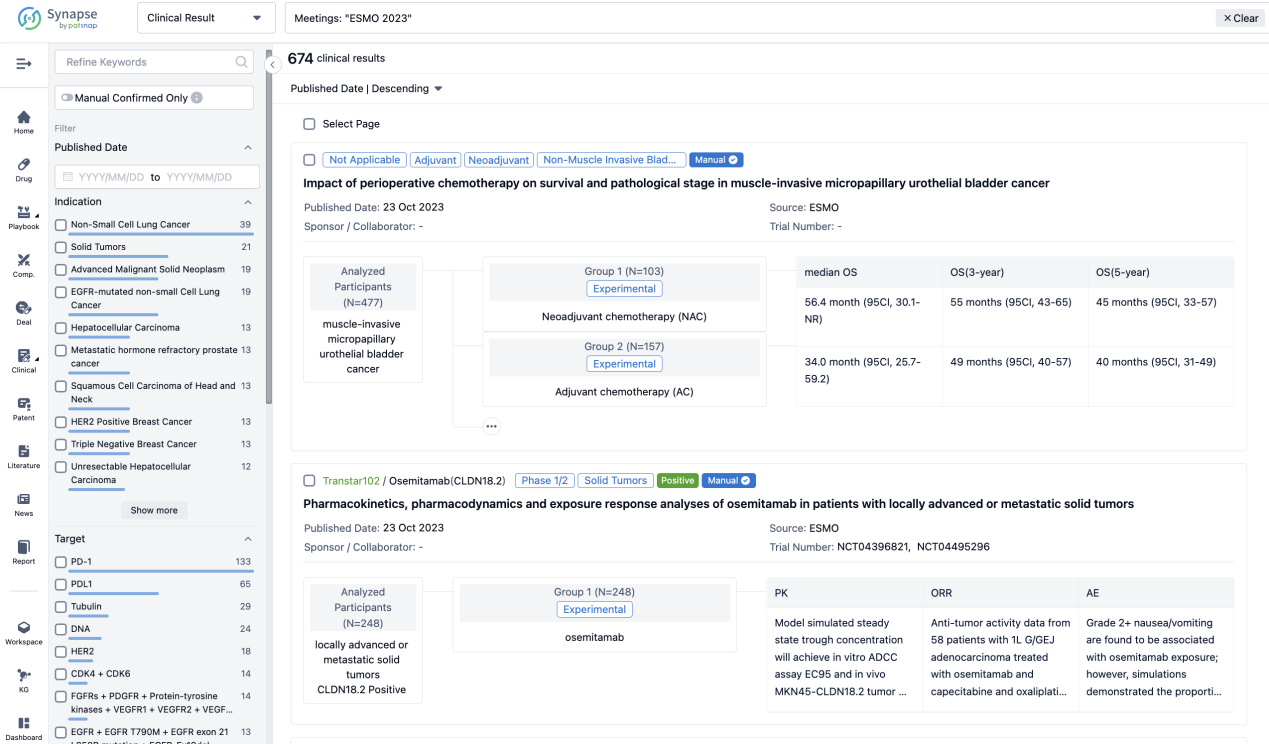
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.

Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!