May 2025 Patent Highlights: Vertex’s NaV Inhibitors
On January 30, 2025, the U.S. Food and Drug Administration (FDA) approved JOURNAVX (suzetrigine), a first-in-class oral, selective, non-opioid inhibitor of the NaV1.8 voltage-gated sodium channel isoform, for the treatment of moderate to severe acute pain in adults. According to Vertex Pharmaceuticals' Q1 financial report, as of April 18, hospitals and retail pharmacies had issued and dispensed over 20,000 prescriptions for suzetrigine across various acute pain conditions. Given the robust sales projections for suzetrigine, Vertex Pharmaceuticals has positioned it as a potential blockbuster product.
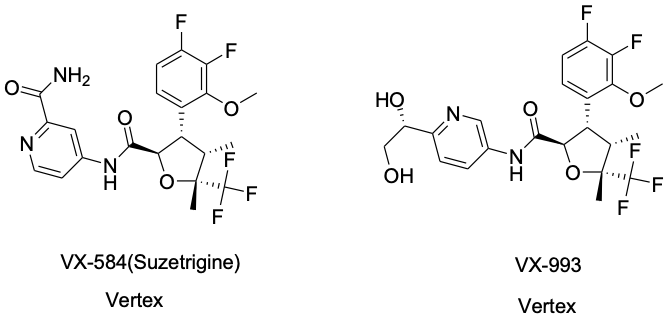
Suzetrigine is the first non-opioid novel pain medication approved in over two decades for pain management; however, its efficacy appears to be limited. Consequently, Vertex Pharmaceuticals is actively developing a second-generation NaV1.8 inhibitor. VX-993, Vertex’s next-generation selective NaV1.8 inhibitor, has received Fast Track designation from the U.S. Food and Drug Administration (FDA) for the treatment of moderate-to-severe acute pain in both its oral and intravenous formulations. Vertex anticipates completing the Phase II clinical trial for the oral formulation of VX-993 this quarter, with results expected in the second half of 2025. Additionally, Vertex has successfully completed a Phase I clinical trial for the intravenous formulation of VX-993 in healthy volunteers.
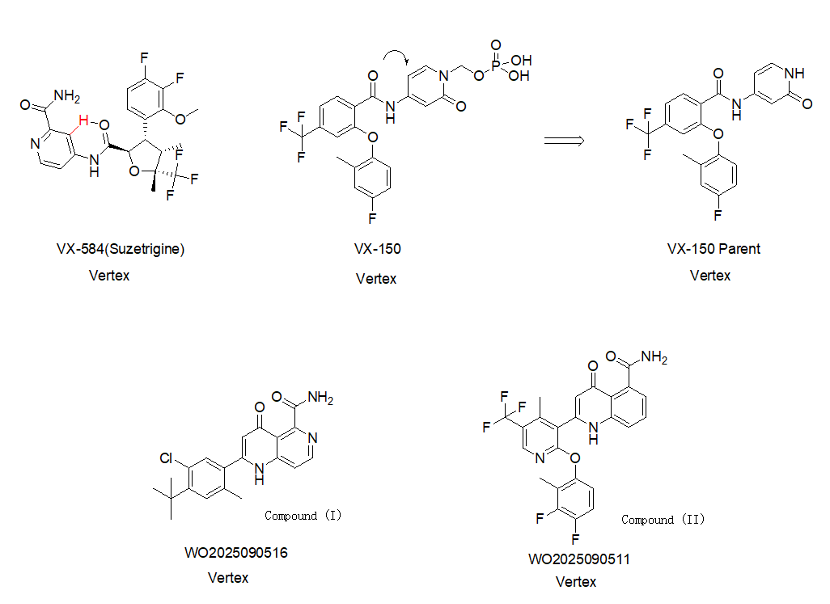
Vertex Pharmaceuticals continues to advance its product development plans in the field of NaV inhibitors. On May 1, Vertex Pharmaceuticals disclosed two patent applications, WO2025090516A1 and WO2025090511A1, which cover the preparation methods and crystalline forms of two compounds, Compound I and Compound II, as well as their pharmaceutically acceptable salts, solvates, hydrates, co-crystals, pharmaceutical compositions containing them, and methods of using them for the treatment of pain. Additionally, two further patent applications, WO2025090480 and WO2025090465, were disclosed, covering NaV inhibitors for the treatment of pain. These compounds appear to represent modifications and innovations based on closed-ring analogs of VX-584 and fragments of VX-150.
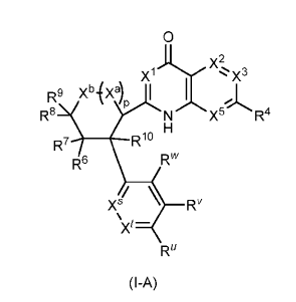
WO2025090465
Vertex
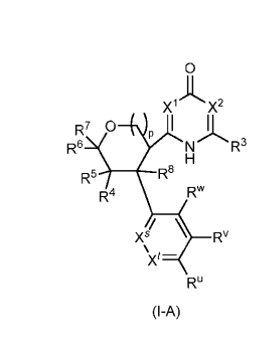
WO2025090480
Vertex
Vertex Pharmaceuticals announced its intention to continue researching and developing a series of potential drug candidates for the treatment of acute and chronic pain, including chronic neuropathic pain. However, apart from VX-993, Vertex Pharmaceuticals has not disclosed any additional drug candidates in the analgesic field, which limits the ability to assess the value of these new product patents for the company. More concerning is the fact that the compounds disclosed in WO2025090480 and WO2025090465 provide only a range of activity data, making it challenging to evaluate their advantages or clinical potential relative to VX-584.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!