Mediar Therapeutics Starts New Clinical Trial for Innovative Anti-Fibrosis Antibodies
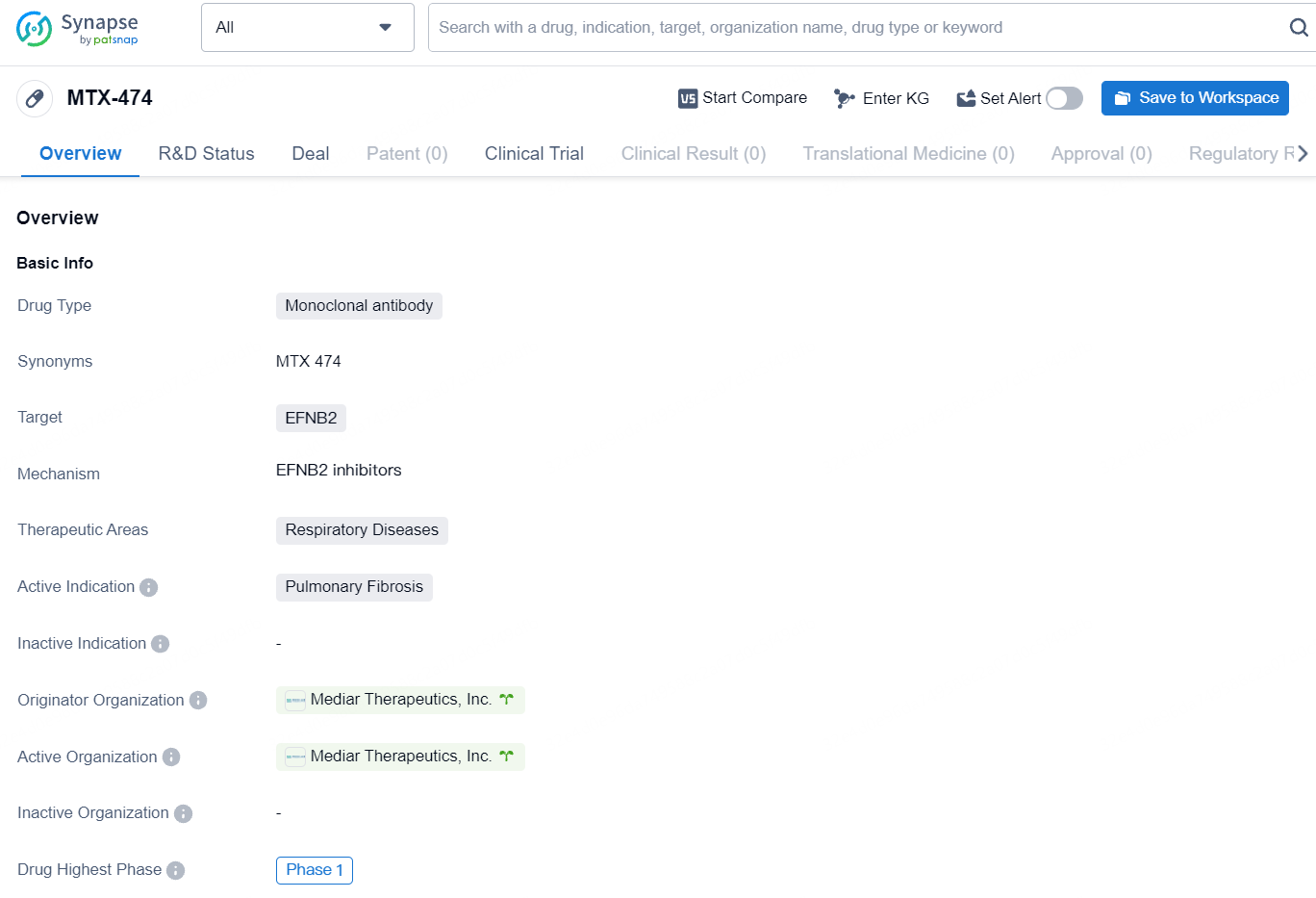
Mediar Therapeutics, Inc., a biotechnology firm at the clinical stage developing an innovative range of therapies aimed at stopping fibrosis progression, has revealed that the initial group of participants has received doses in a Phase 1 trial. This study is assessing the safety and tolerability of MTX-474, a human IgG1 antibody created to inhibit EphrinB2 signaling.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The research conducted in Australia aims to evaluate the safety, tolerability, pharmacokinetics, and target engagement of MTX-474 in healthy volunteers through staggered single ascending dose and multiple ascending dose cohorts.
The company also highlights progress in its US Phase 1 trial of the WISP1-targeting antibody, MTX-463, with about 75% of cohorts dosed, and anticipates completing the trial by the end of 2024. Additionally, MTX-463 has received orphan drug and fast track designations from the FDA, highlighting WISP1's potential to meet critical unmet needs in treating rare fibrotic diseases, such as Idiopathic Pulmonary Fibrosis.
“I am enthusiastic about the growing clinical portfolio at Mediar and its innovative approach to directly target the myofibroblast, the main pathogenic cell in fibrosis,” stated Toby Maher, M.D., Ph.D., Professor of Medicine and Director of Interstitial Lung Diseases at the University of Southern California, and member of Mediar’s Clinical Advisory Board. “Both the EphrinB2 and WISP1 antibody programs present potentially novel methods to halt and possibly reverse fibrosis. I anticipate the completion of the Phase 1 studies and the planned future Phase 2 trials in systemic sclerosis and IPF, respectively.”
“We are pleased to see our first-in-class programs progressing from compelling science to clinical trials,” said Rahul Ballal, Ph.D., Chief Executive Officer of Mediar Therapeutics. “Initiating dosing in our MTX-474 trial signifies another key step forward for Mediar and, along with our ongoing Phase 1 study of MTX-463, opens new potential avenues for targeting fibrosing diseases. Furthermore, the FDA's designation of orphan drug and fast track status for MTX-463 underscores the potential of this innovative therapy to address the substantial unmet need in IPF.”
MTX-474 is a first-in-class human IgG1 antibody designed to inhibit the EphrinB2 signaling pathway, which contributes to the onset and progression of fibrosis. Ephrin ligands and Eph receptors regulate biological processes implicated in tissue fibrosis, including cell migration, myofibroblast activation, and tissue remodeling. Accumulating evidence has linked EphrinB2 to fibrosis in the skin, lungs, and heart. The expression levels of EphrinB2 and its receptors are measurable in human blood and correlate with disease severity.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
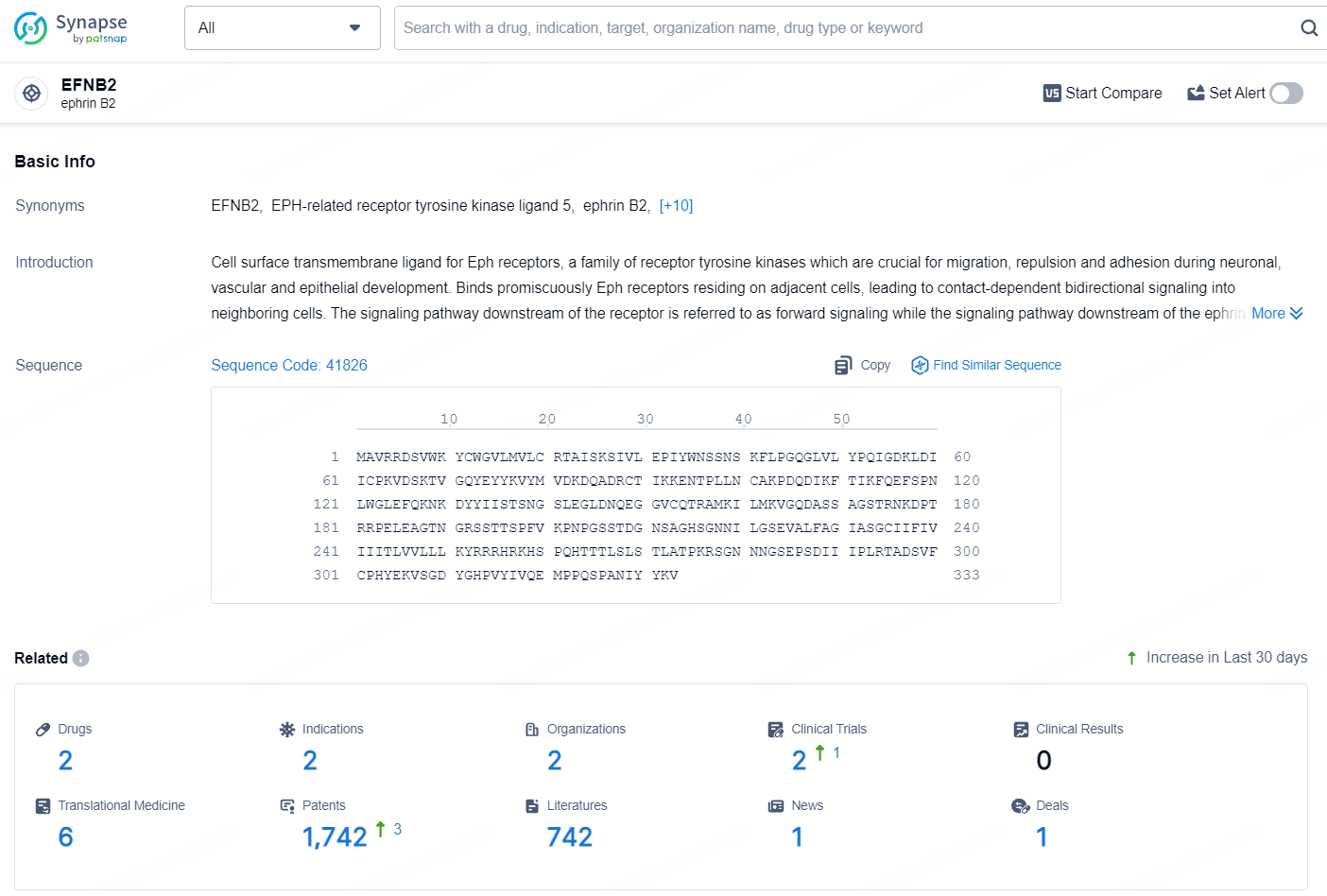
According to the data provided by the Synapse Database, As of August 12, 2024, there are 2 investigational drugs for the EphrinB2 targets, including 2 indications, 2 R&D institutions involved, with related clinical trials reaching 2, and as many as 1742 patents.
As the drug is currently in the Phase 1 stage, further clinical data and progress are awaited to determine its efficacy and safety profile. However, the development of MTX-474 marks a notable advancement in the pursuit of novel therapeutic options for respiratory diseases, particularly in the realm of pulmonary fibrosis.