NeuroBo, Dong-A ST, & ImmunoForge Collaborate on Monthly Obesity Treatment DA-1726
NeuroBo Pharmaceuticals, Inc., a biotechnology enterprise in the clinical stages, dedicated to advancing treatments for cardiometabolic disorders, has declared the signing of a collaborative research agreement with Dong-A ST Co. Ltd. and ImmunoForge. The collaboration aims to develop a long-acting, once-monthly formulation of DA-1726, a new dual oxyntomodulin analog agonist that targets both the glucagon-like peptide-1 receptor (GLP1R) and the glucagon receptor (GCGR).
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Hyung Heon Kim, President and CEO of NeuroBo, announced, “The execution of this research agreement, in partnership with Dong-A ST and ImmunoForge, marks an important step towards the potential creation of a long-acting version of DA-1726. This would improve patient adherence and simplify the administration process for obesity treatment.” He further explained, “We are optimistic that ImmunoForge’s ELP platform technology could transform DA-1726, currently undergoing Phase 1 trials, into a once-a-month injection, addressing the challenges of converting peptides like DA-1726 into extended-release formats. We anticipate close cooperation with Dong-A ST and ImmunoForge to develop a pioneering, monthly obesity treatment option.”
Sung-Min Ahn and Kiho Chang, Co-CEOs of ImmunoForge, added, “This partnership with top-tier pharmaceutical leader Dong-A ST and NeuroBo highlights the promise of our ELP platform technology. Our patented once-monthly, long-acting ELP platform technology has the potential to extend a drug's half-life up to 200-fold, and we are eager to investigate its potential with NeuroBo’s DA-1726, a promising approach for obesity treatment.”
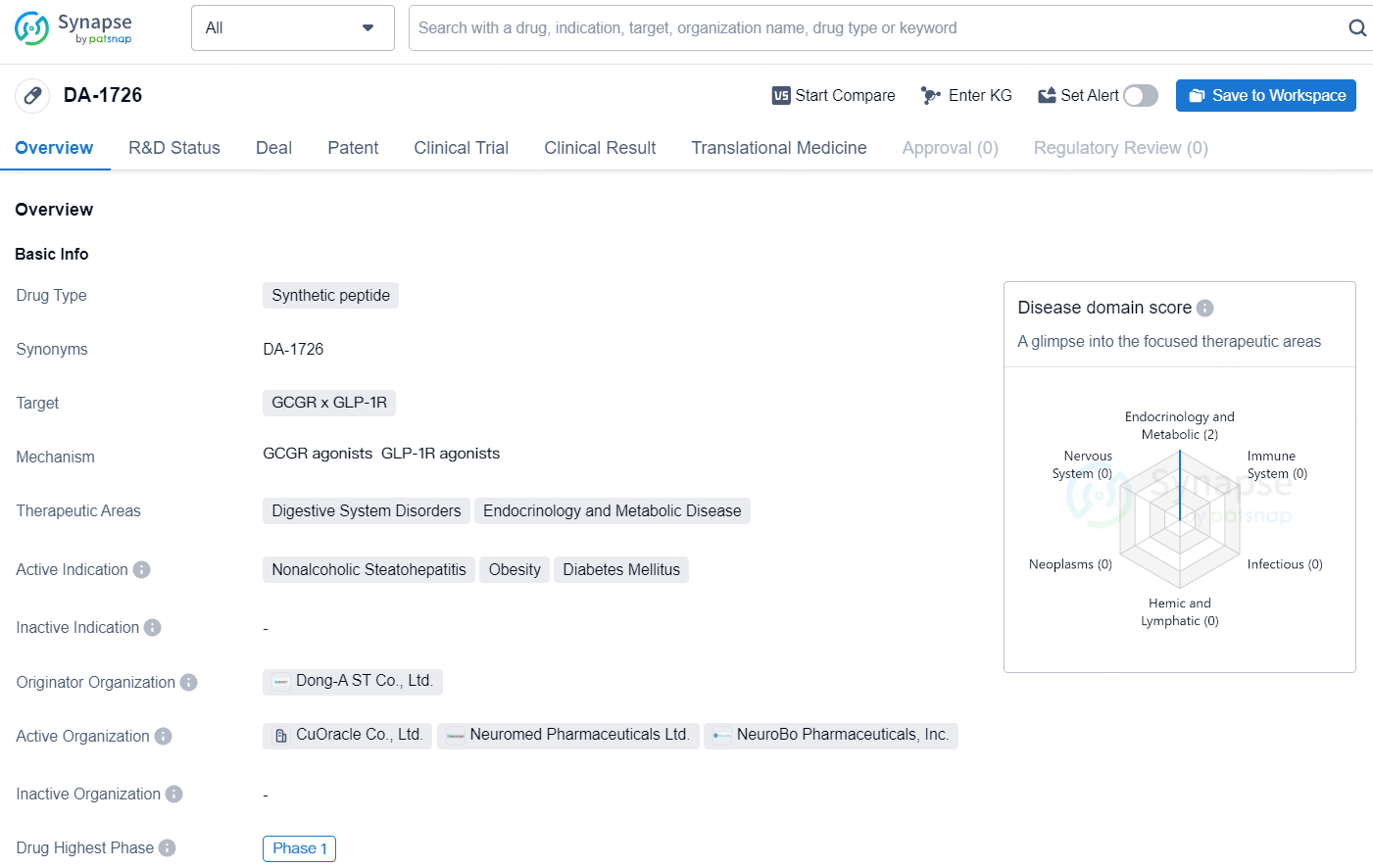
DA-1726 is a novel oxyntomodulin analogue acting as a GLP1R/GCGR dual agonist, designated for weekly subcutaneous administration for addressing obesity and Metabolic Dysfunction-Associated Steatohepatitis. This dual agonist of GLP-1 and glucagon receptors promotes weight reduction by decreasing appetite and boosting energy use. Pre-clinical mouse studies have shown that DA-1726 leads to better weight loss than semaglutide and cotadutide. Additionally, compared to tirzepatide and survodutide in pre-clinical mouse models, DA-1726 demonstrated equivalent weight reduction with higher food intake, while also preserving lean mass and showing superior lipid-lowering effects compared to survodutide.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
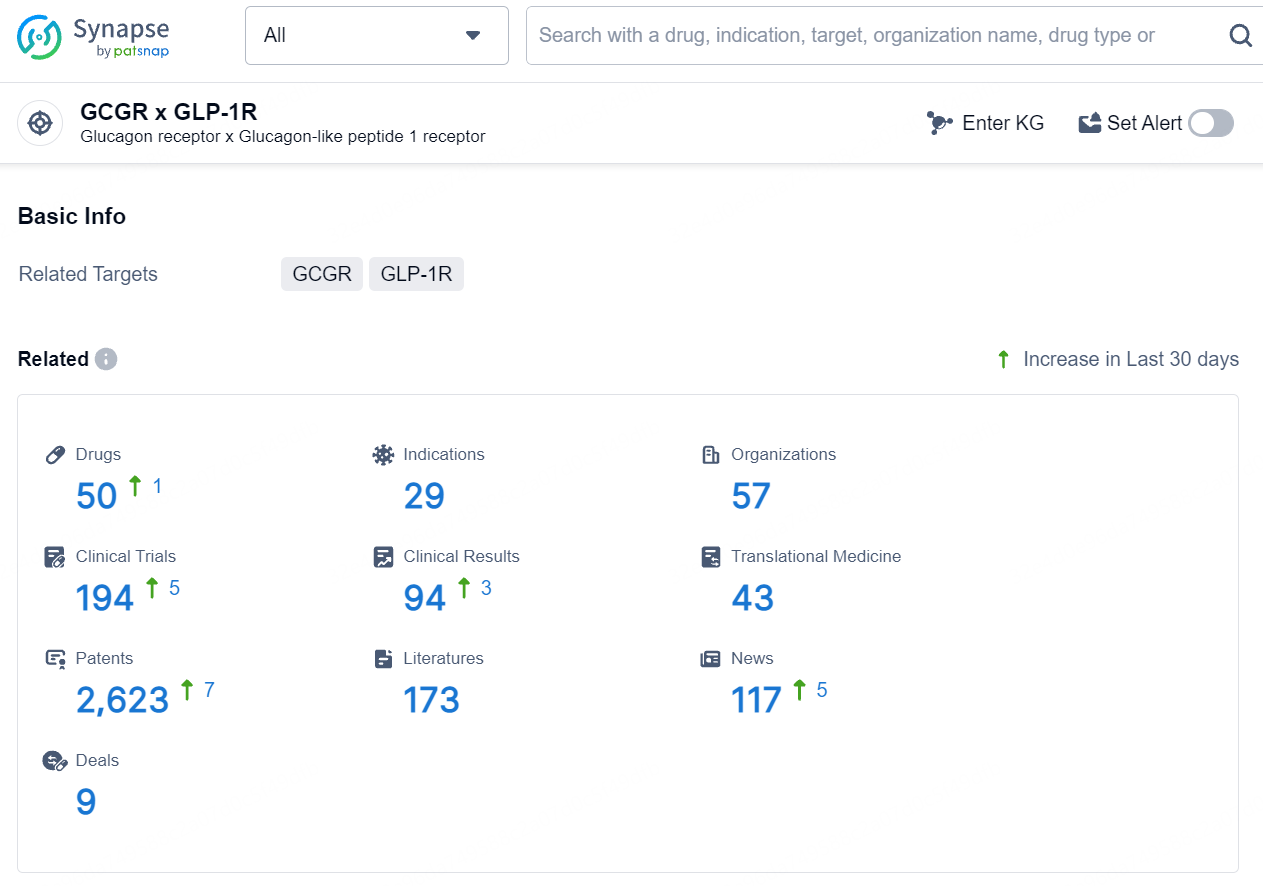
According to the data provided by the Synapse Database, As of August 9, 2024, there are 50 investigational drugs for the GLP1R/GCGR targets, including 29 indications, 57 R&D institutions involved, with related clinical trials reaching 194, and as many as 2623 patents.
DA-1726 is a synthetic peptide drug with a focus on addressing digestive system disorders, endocrinology, and metabolic diseases. The drug is currently in Phase 1 of development and is being investigated for its potential to treat nonalcoholic steatohepatitis, obesity, and diabetes mellitus. Further research and clinical trials are necessary to determine the efficacy and safety of DA-1726 in addressing these medical conditions.