MediLink Reveals Worldwide Partnership and Supply Deal for YL201 Combo Treatment
MediLink Therapeutics (Suzhou) Co., Ltd. ("MediLink"), a biotech company in the clinical stage, announced a partnership for a global clinical trial and a supply agreement with Amgen Inc. Under this agreement, Amgen will conduct an international clinical trial to explore the treatment potential of combining MediLink’s B7-H3-targeting antibody-drug conjugate (ADC) YL201 with Amgen’s DLL3- and CD3-targeting bispecific T-cell engager (BiTE®) IMDELLTRA™ in patients with extensive-stage small cell lung cancer (ES-SCLC). MediLink will supply the YL201 investigational drug for the study.
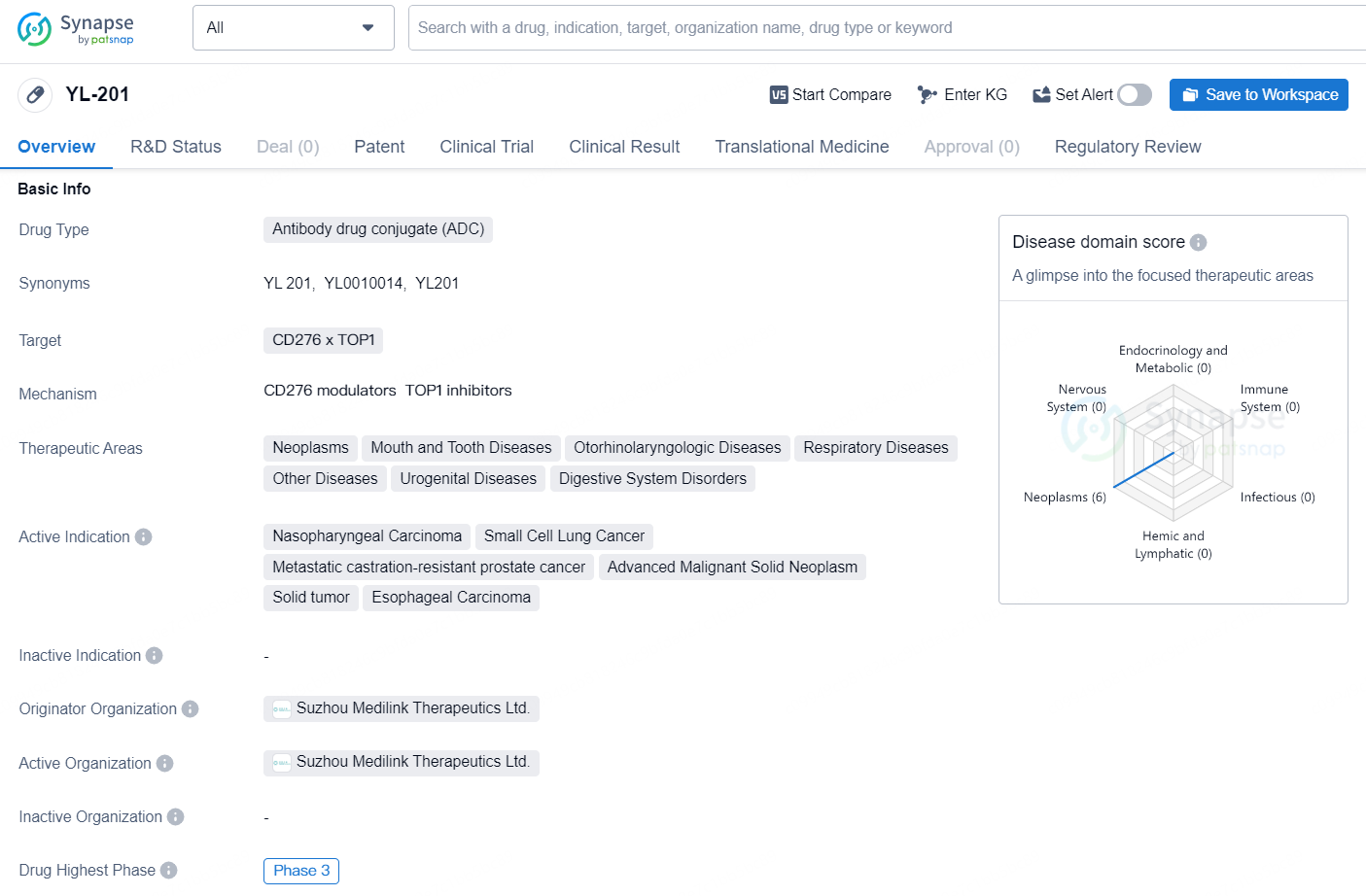
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
This global, multi-center, open-label Phase Ib clinical trial aims to assess the safety, tolerability, pharmacokinetics, and effectiveness of this combination treatment in patients with ES-SCLC. Both YL201 and IMDELLTRA™ have demonstrated promise in treating ES-SCLC. In May this year, the FDA granted IMDELLTRA™ accelerated approval, and it is currently available in the US for adult patients with ES-SCLC whose disease has progressed after receiving platinum-based chemotherapy.
This approval was given based on the overall response rate and the duration of the response. Continued approval for this indication may depend on the verification and description of clinical benefit in one or more confirmatory trials. The efficacy of YL201 as a standalone treatment is promising in ES-SCLC. MediLink has disclosed data from a Phase I/II clinical trial of YL201 in patients with advanced solid tumors, including SCLC, as a selected oral presentation at the ESMO Congress 2024. This collaboration in clinical trials seeks to investigate the potential of these two innovative drugs for the treatment of ES-SCLC, providing a novel and synergistic mechanism of action for clinical benefits.
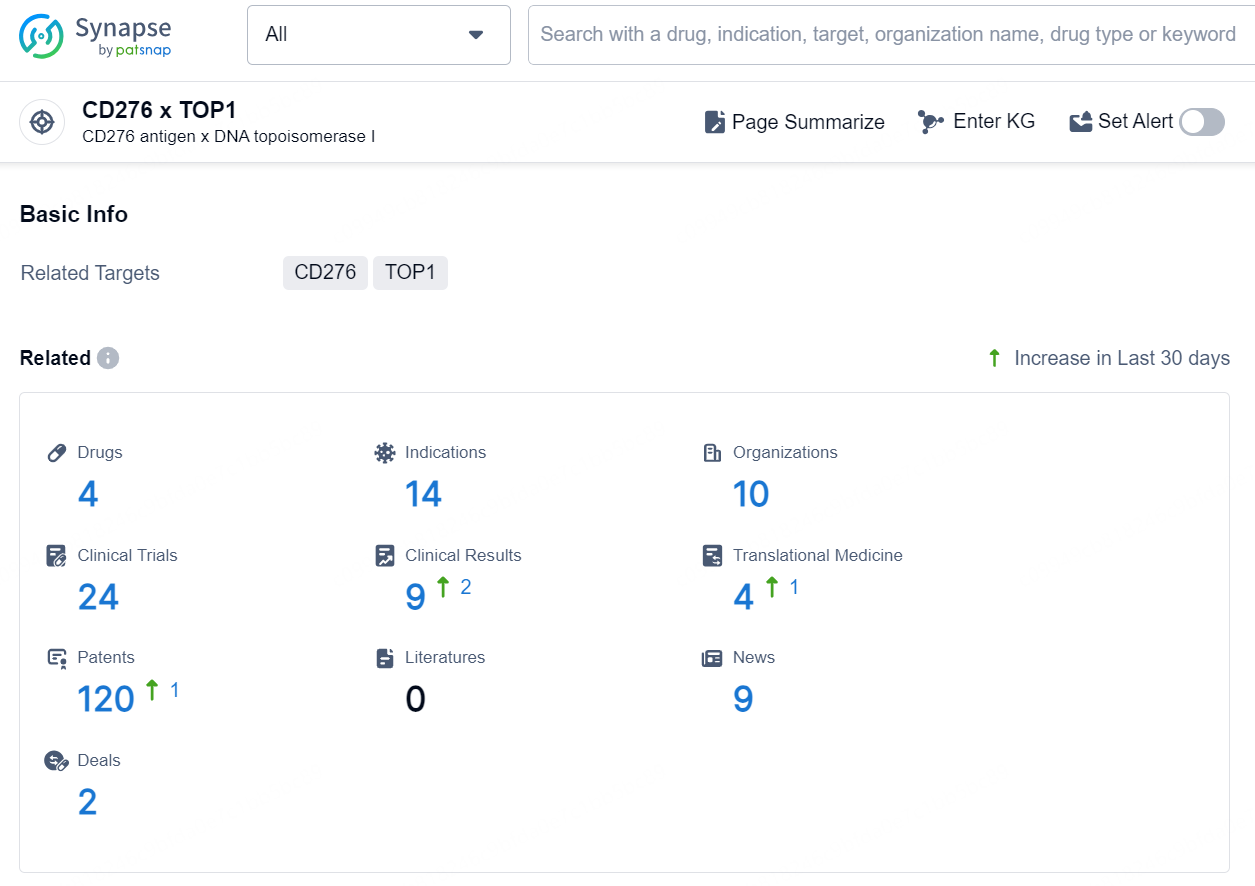
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of October 10, 2024, there are 27 investigational drugs for the CD276 x TOP1 target, including 41 indications, 35 R&D institutions involved, with related clinical trials reaching 54, and as many as 1773 patents.
The drug YL-201 is an antibody drug conjugate (ADC) that targets CD276 x TOP1. It is being developed for the treatment of various neoplastic diseases, as well as mouth and tooth diseases, otorhinolaryngologic diseases, respiratory diseases, urogenital diseases, digestive system disorders, and other diseases. The drug's active indications include nasopharyngeal carcinoma, small cell lung cancer, metastatic castration-resistant prostate cancer, advanced malignant solid neoplasm, solid tumor, and esophageal carcinoma.