TiumBio Reports Initial Patient Dosing in Phase 2 Trial for TU2218, an Oral Immuno-Oncology Medication
TiumBio Co., Ltd. (Kosdaq: 321550), a biopharmaceutical company in the clinical-stage dedicated to the discovery and development of novel treatments for patients with rare and incurable conditions, announced that it has administered the first dose to a patient in its Phase 2 clinical study of TU2218.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
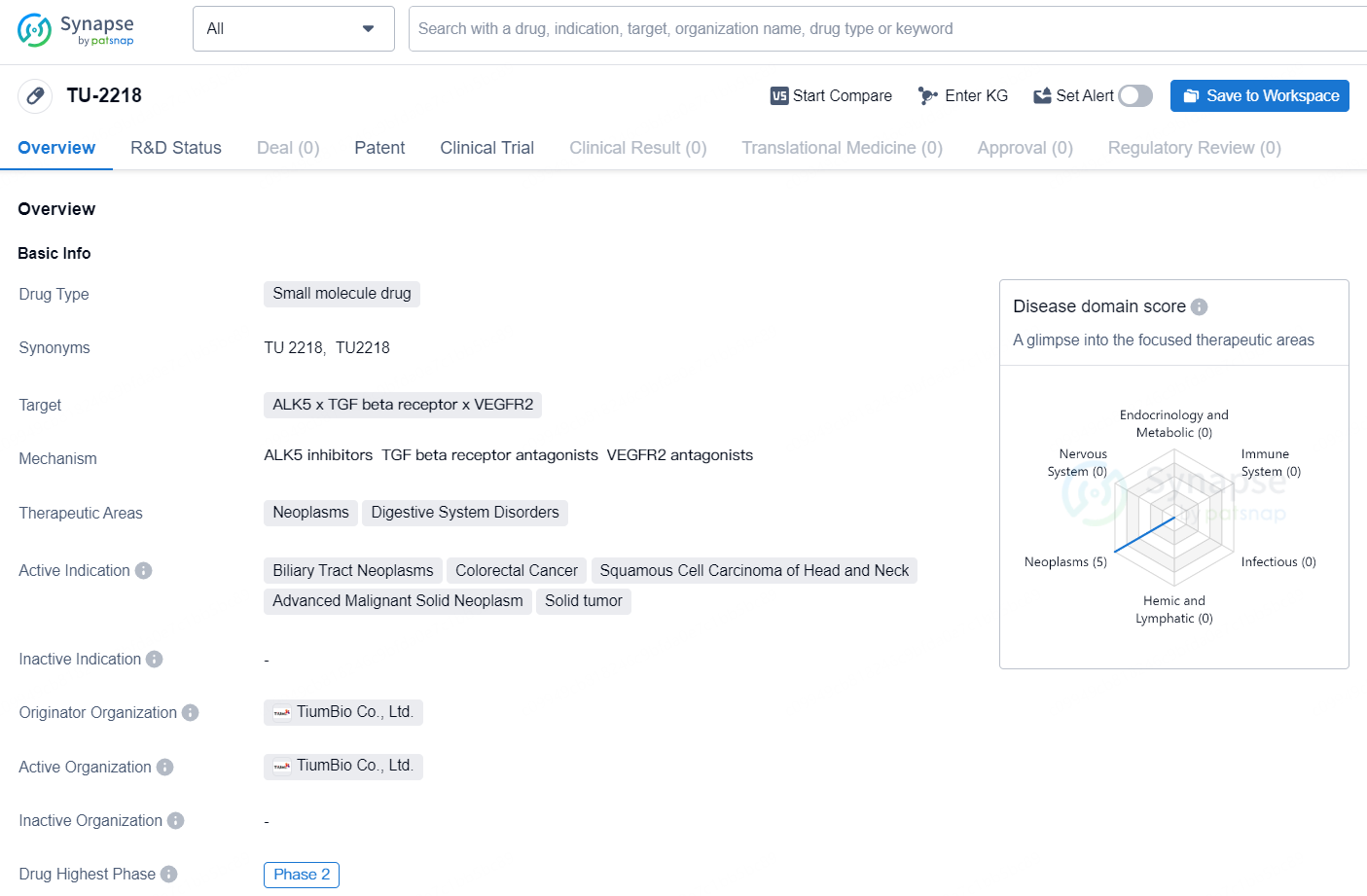
TU2218 is an innovative oral dual inhibitor that targets TGFR1 and VEGFR2. It is known that the TGF-ß and VEGF pathways can inhibit the effectiveness of immune checkpoint inhibitors (ICIs), so by blocking these two pathways, TU2218 may enhance the performance of ICIs.
In Phase 1a and 1b clinical trials, TiumBio assessed the safety, pharmacokinetics, and pharmacodynamics of TU2218 both as a standalone treatment and in combination with Keytruda (pembrolizumab) in a group of 41 patients with advanced solid tumors. The resulting data was used to identify dose levels for subsequent Phase 2 trials. The Phase 2a trial aims to evaluate the safety and effectiveness of TU2218 in concert with Keytruda in patients suffering from head and neck squamous cell carcinoma (HNSCC), biliary tract cancer (BTC), and colorectal cancer (CRC).
The Phase 2 trial kicks off at Seoul National University Hospital and Asan Medical Center in South Korea, with plans to broaden to additional medical centers in the United States. The initial dose was given to a patient with HNSCC.
HNSCC describes cancerous tumors found in the oral cavity, throat, larynx, or salivary glands, with the typical treatment involving surgery and radiation. According to Global Data, the worldwide number of HNSCC patients is projected to reach roughly 610,000 by 2023 and could surpass 670,000 by 2030.
"HNSCC presents a large unmet medical need, given that the average survival rate for first-line therapies is typically about one year," stated Hun-taek Kim, Ph.D., MBA, CEO of TiumBio. "The cancer types for the Phase 2 clinical trial were chosen based on other trials that showed significant anti-cancer effects from targeting the TGF-ß or VEGF pathways. Our objective is to establish TU2218 as a first-line therapy for HNSCC," he noted.
In the Phase 1b trial, out of 10 patients with advanced solid tumors who were given a 195mg dose daily (the dose chosen for Phase 2) of TU2218 in combination with Keytruda, three patients achieved a partial response (PR) and five maintained stable disease (SD), resulting in an 80% disease control rate (DCR).
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
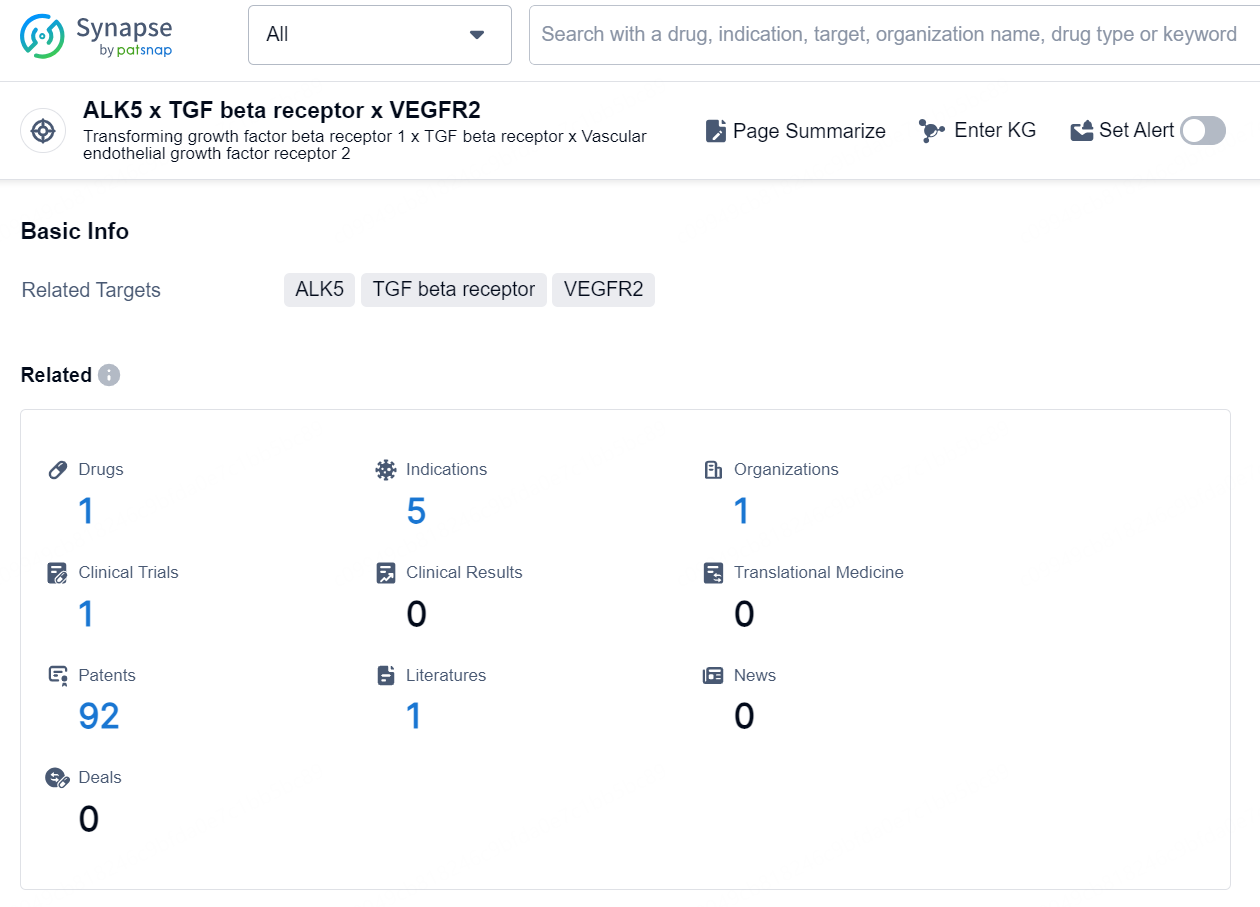
According to the data provided by the Synapse Database, As of October 9, 2024, there are 1 investigational drug for the ALK5 x TGF beta receptor x VEGFR2 targets, including 5 indications, 1 R&D institution involved, with related clinical trial reaching 1, and as many as 92 patents.
TU-2218 is a small molecule drug developed by TiumBio Co., Ltd. The drug targets ALK5, TGF beta receptor, and VEGFR2 and is primarily intended for the treatment of neoplasms and digestive system disorders. The active indications for [TU-2218] include biliary tract neoplasms, colorectal cancer, squamous cell carcinoma of head and neck, advanced malignant solid neoplasm, and solid tumor.