Melphalan Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Melphalan's R&D Progress
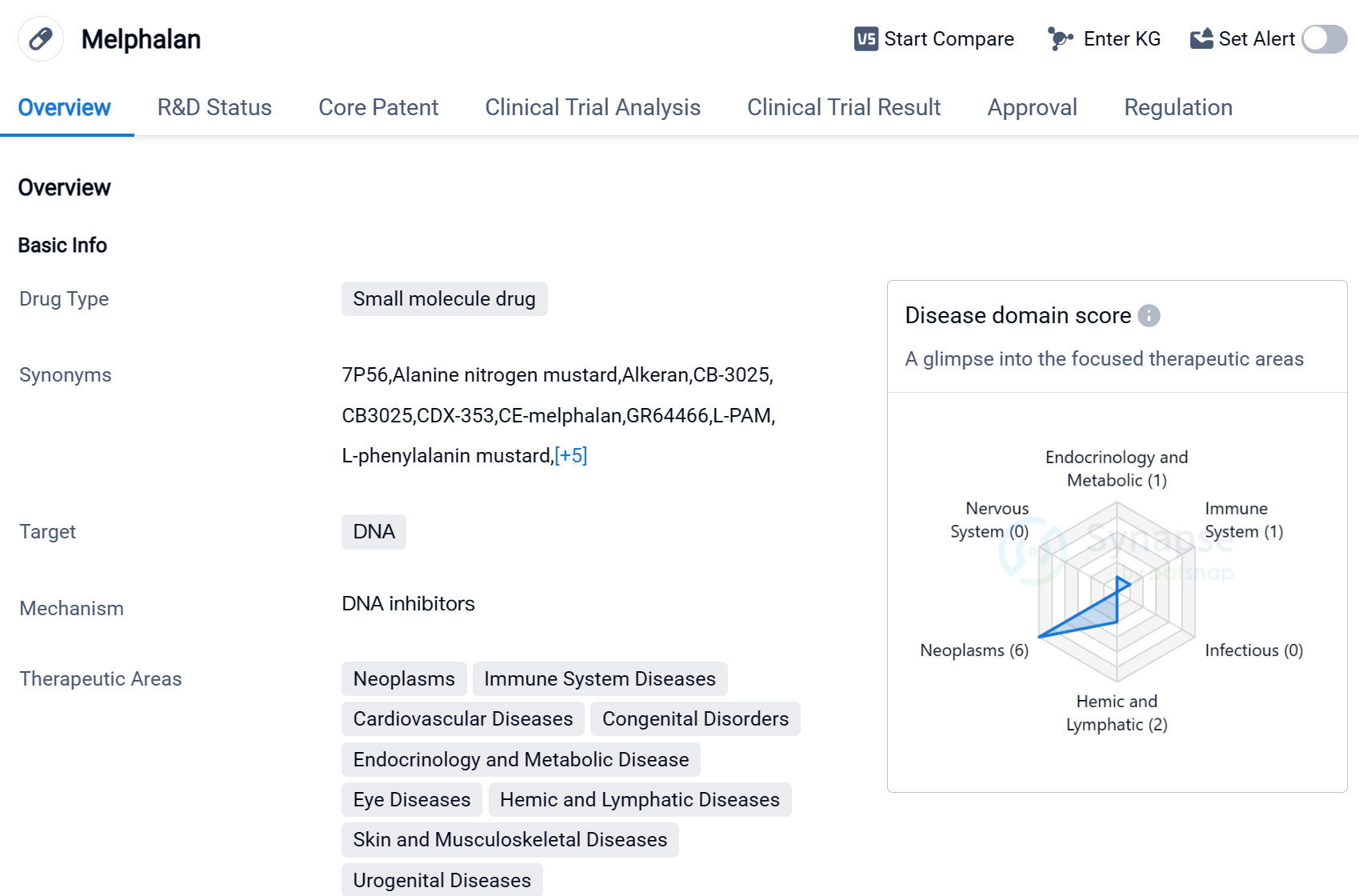
Melphalan is a small molecule drug that primarily targets DNA. It has been approved for use in various therapeutic areas, including neoplasms, immune system diseases, cardiovascular diseases, congenital disorders, endocrinology and metabolic disease, eye diseases, hemic and lymphatic diseases, skin and musculoskeletal diseases, and urogenital diseases.
The drug has shown efficacy in treating several active indications, such as polycythemia vera, retinoblastoma, breast cancer, melanoma, multiple myeloma, and ovarian cancer. Melphalan's ability to target DNA makes it a valuable tool in combating these diseases, as it can disrupt the replication and growth of cancer cells.
Melphalan was first approved for use in 1963 in Canada, making it a well-established drug with a long history of use. Its originator organization is GSK Plc, a renowned pharmaceutical company known for its contributions to the healthcare industry.
In terms of regulatory status, Melphalan has received priority review and orphan drug designation. Priority review is a regulatory pathway that expedites the review process for drugs that address unmet medical needs, while orphan drug designation is granted to drugs that treat rare diseases.
Melphalan has achieved the highest phase of approval globally, indicating its widespread acceptance and recognition in the medical community.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Melphalan: DNA inhibitor
DNA inhibitors are substances that interfere with the replication or transcription of DNA molecules. From a biomedical perspective, DNA inhibitors are often used in cancer treatment to prevent the growth and division of cancer cells. By targeting and inhibiting specific enzymes or proteins involved in DNA replication or transcription, these inhibitors can disrupt the ability of cancer cells to proliferate. Some examples of DNA inhibitors used in cancer therapy include topoisomerase inhibitors, which prevent the unwinding of DNA strands, and nucleoside analogs, which interfere with the incorporation of nucleotides into DNA chains. These inhibitors are designed to selectively target cancer cells while minimizing damage to healthy cells.
Drug Target R&D Trends for Melphalan
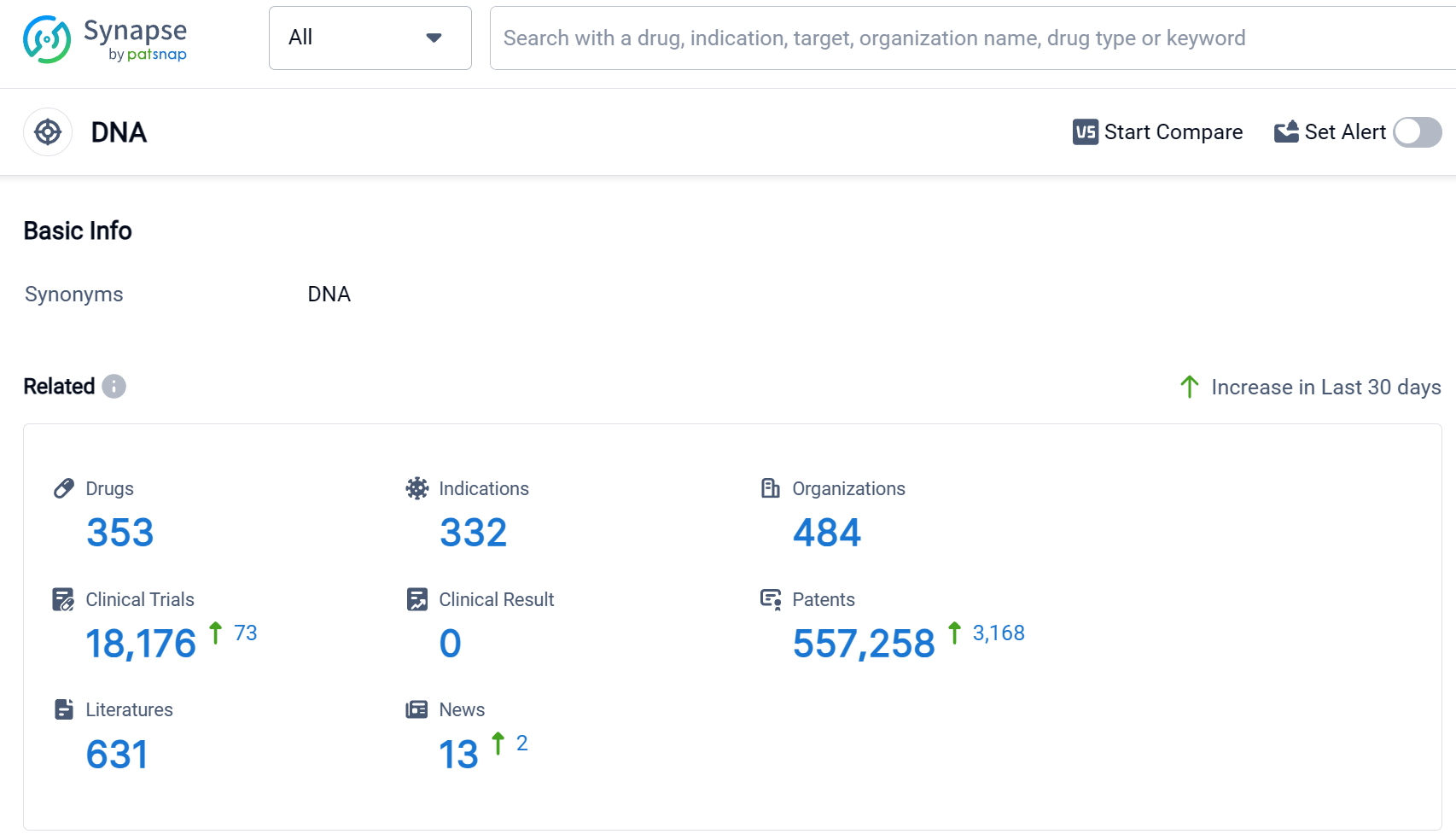
According to Patsnap Synapse, as of 9 Sep 2023, there are a total of 353 DNA drugs worldwide, from 484 organizations, covering 332 indications, and conducting 18176 clinical trials.
The analysis of the current competitive landscape and future development of target DNA reveals that Pfizer Inc., Novartis AG, Baxter International, Inc., Takeda Pharmaceutical Co., Ltd., and Johnson & Johnson are the companies growing fastest under the current target. The most common indications for drugs targeting the DNA include lymphoma, ovarian cancer, breast cancer, neoplasms, and Hodgkin's lymphoma. Small molecule drugs, monoclonal antibodies, and ADCs are progressing most rapidly under the current targets. China, the United States, Japan, and the European Union are the countries/locations developing fastest under the current targets, with China showing significant progress. Overall, the target DNA presents a promising opportunity for the development of innovative drugs in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Melphalan is a small molecule drug that targets DNA and has been approved for use in various therapeutic areas. It has shown effectiveness in treating several active indications, including polycythemia vera, retinoblastoma, breast cancer, melanoma, multiple myeloma, and ovarian cancer. With its long history of use since its approval in 1963 in Canada, Melphalan has established itself as a valuable tool in the fight against various diseases. Its regulatory status as a priority review and orphan drug further highlights its significance in addressing unmet medical needs and rare diseases.