Mim8 Outperforms On-Demand Treatments in Haemophilia A Patients
Novo Nordisk released findings from the phase 3 FRONTIER2 study involving 254 adults and adolescents aged 12 and older with haemophilia A, both with and without inhibitors. The study evaluated prophylactic treatment regimens of Mim8 administered either weekly or monthly. These results were shared at the International Society of Thrombosis and Haemostasis Annual Congress held in Bangkok, Thailand.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"Despite strides in treatment, numerous unmet needs persist for those with haemophilia A," stated Dr. Maria Elisa Mancuso, the principal investigator of the FRONTIER2 study and Senior Consultant in Haematology at the Centre for Thrombosis and Haemorrhagic Diseases, IRCCS Humanitas Research Hospital, Milan, Italy. "The data from the FRONTIER2 study illustrated that Mim8 prophylaxis was superior to both on-demand treatment and previous prophylactic clotting factor therapies in reducing the number of treated bleeding episodes in haemophilia A patients, regardless of their inhibitor status. It's also promising that up to 95% of participants in certain subgroups who received Mim8 reported no bleeding episodes."
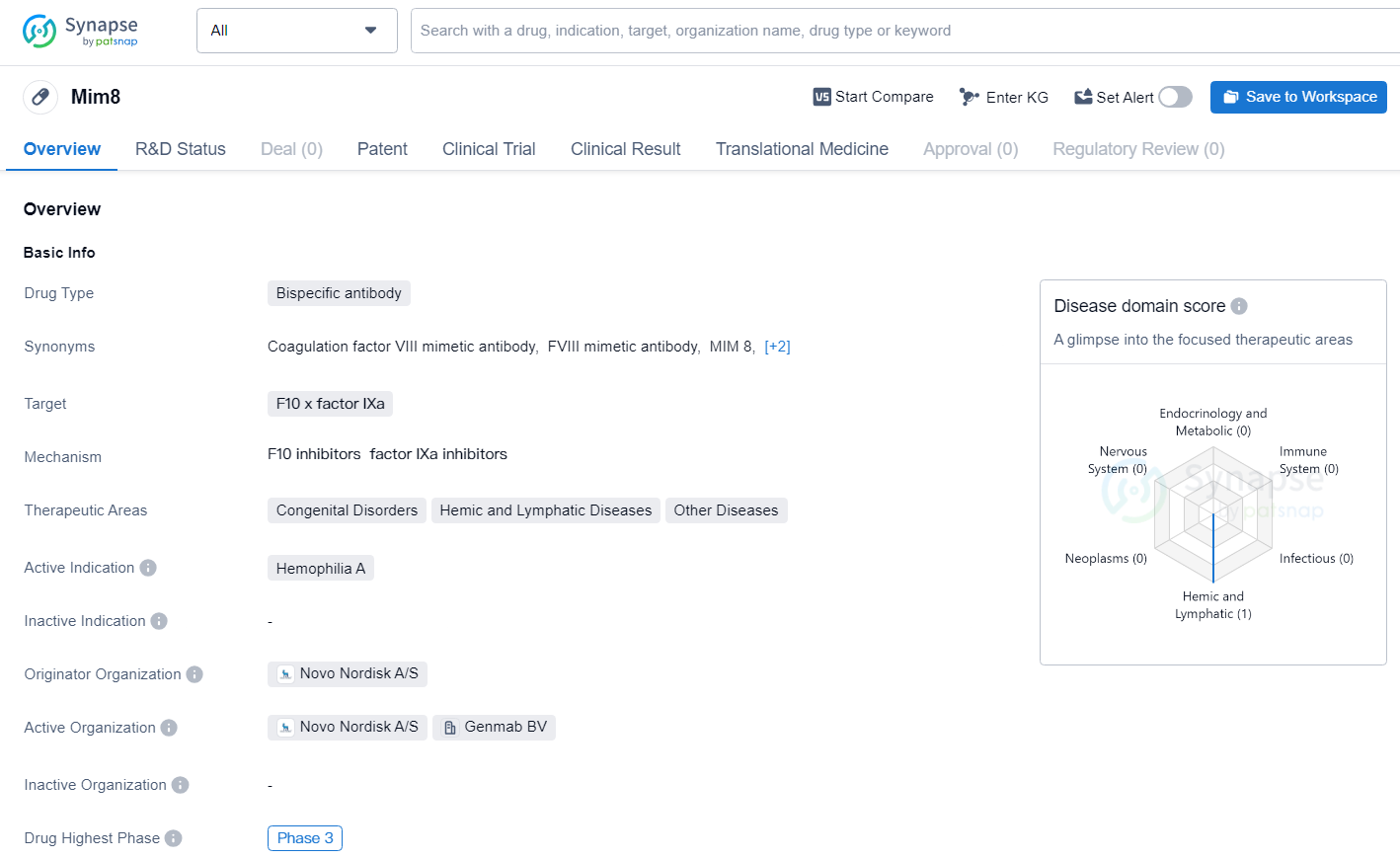
Mim8 is an advanced Factor VIIIa (FVIIIa) mimetic bispecific antibody, crafted to potentially offer continuous haemostasis. It is under development as a weekly or monthly prophylactic treatment for haemophilia A patients, with and without inhibitors. Mim8 is administered subcutaneously, linking Factor IXa/X (FIXa/FX) and thus compensating for the missing FVIII.
"The efficacy and safety data from FRONTIER2 are highly promising," remarked Martin Holst Lange, Executive Vice President of Development at Novo Nordisk. "Mim8 offers the possibility of zero bleeds for a significant number of patients and allows flexible dosing to suit their individual lifestyles and needs. We are eager to engage with regulatory authorities on these findings."
Pending regulatory discussions, Novo Nordisk plans to seek the first regulatory approval for Mim8 by late 2024. The ongoing phase 3 FRONTIER programme data will continue to be presented at future conferences and published throughout 2024 and 2025.
Haemophilia is a rare inherited disorder affecting the blood's clotting ability, vital for stopping bleeding. It is estimated to impact around 1,125,000 individuals globally, with haemophilia A constituting approximately 80-85% of these cases. As a rare X-linked recessive condition, haemophilia typically manifests differently in males and females, with approximately 88% of diagnosed individuals being male.
Different types of haemophilia are identified based on the specific defective or missing clotting factor protein. Haemophilia A results from a deficiency or defect in clotting Factor VIII (FVIII). Some haemophilia patients develop inhibitors, an immune response that neutralizes the effect of replacement clotting factors, rendering the therapy ineffective. Current estimates suggest that up to 30% of individuals with severe haemophilia A have inhibitors.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
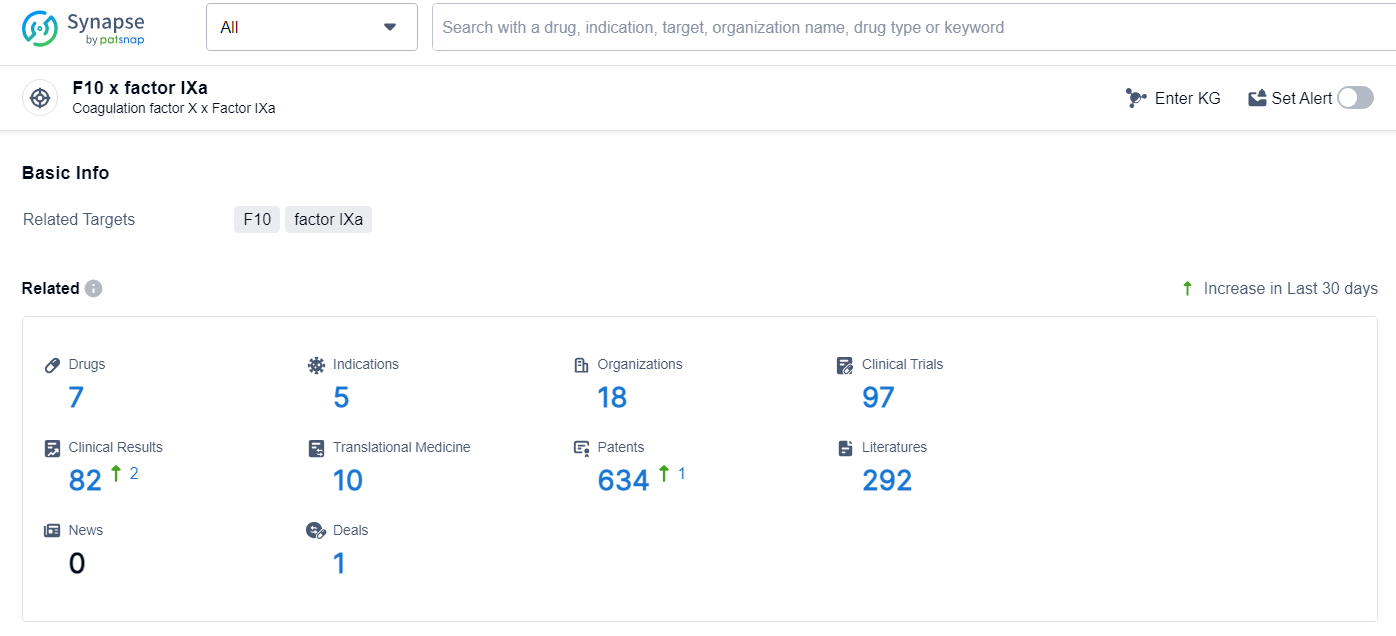
According to the data provided by the Synapse Database, As of June 27, 2024, there are 7 investigational drugs for the F10 and factor IXa target, including 5 indications, 18 R&D institutions involved, with related clinical trials reaching 97, and as many as 634 patents.
Mim8 is a bispecific antibody targeting F10 x factor IXa, currently in Phase 3 clinical development for the treatment of Hemophilia A. Its originator organization is Novo Nordisk A/S, and it holds promise as a potential therapeutic option for patients with this rare genetic disorder.