Sun Pharma's GL0034 Shows Promising Weight Loss in Obesity at ADA's 84th Session
Sun Pharmaceutical Industries Limited revealed findings from a Phase 1, multiple ascending-dose study that examined the safety, tolerability, pharmacokinetics, and pharmacodynamics of GL0034 (Utreglutide) in obese adults. The results were presented orally at the 84th Scientific Sessions of the American Diabetes Association on June 21, 2024, in Orlando, FL.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Obesity is swiftly becoming a prominent global health issue, leading to a variety of metabolic diseases such as type 2 diabetes mellitus, cardiovascular conditions, and metabolic dysfunction-associated steatohepatitis. Effective interventions are essential for managing obesity and its related comorbid conditions. One of the promising therapeutic options for obesity management is the use of GLP-1 (glucagon-like peptide-1) receptor agonists.
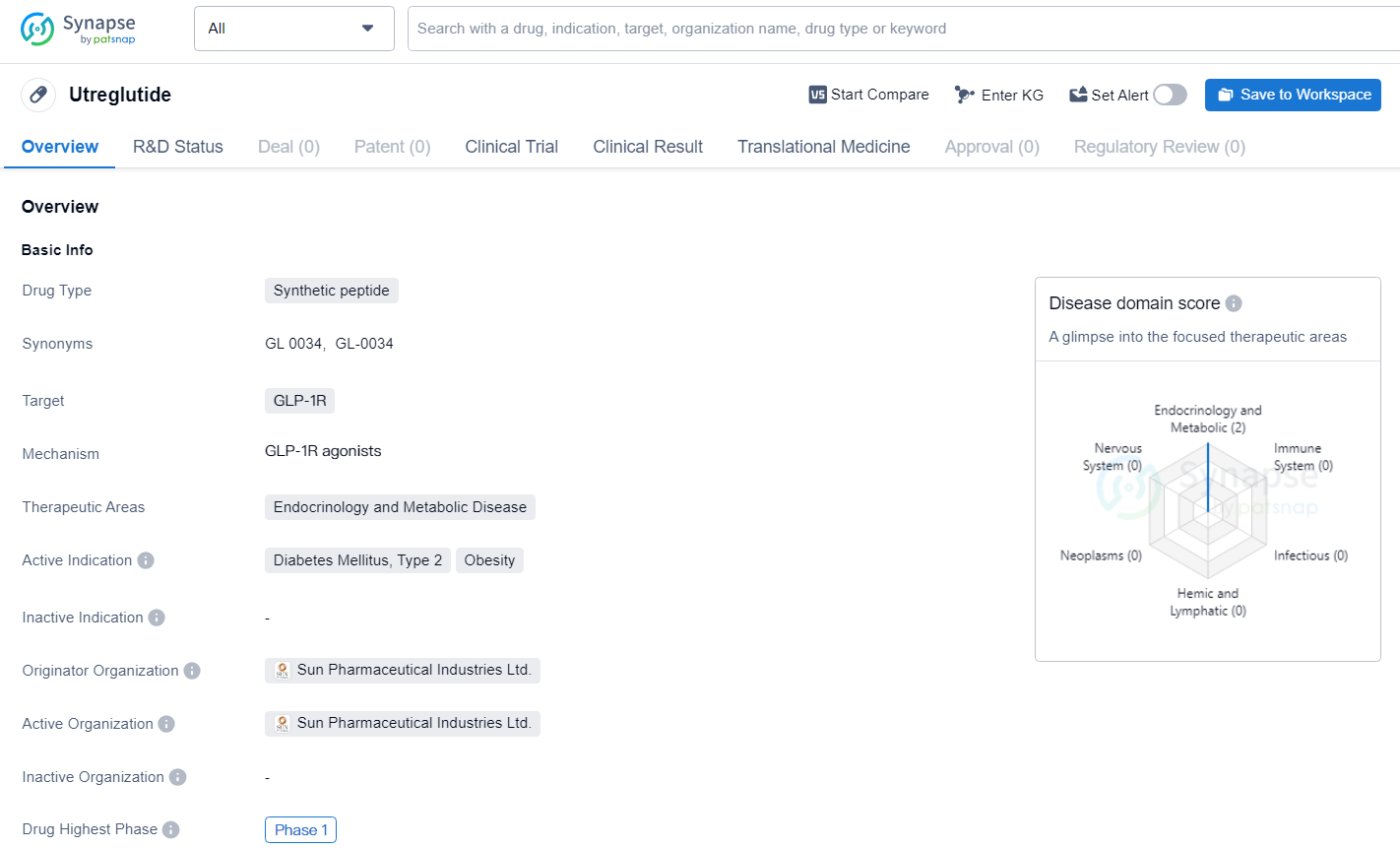
GL0034 (Utreglutide) stands out as a novel incretin analogue with strong, long-lasting agonist effects at the GLP-1 receptor. This cutting-edge compound is currently being investigated for its potential to offer significant clinical benefits beyond mere weight loss and gluco-metabolic improvements in obese individuals.
“The recent findings from the GL0034 study have revealed promising results with considerable weight loss and substantial enhancements in lipid profiles among obese participants,” stated Richard E. Pratley, MD, Medical Director at AdventHealth Diabetes Institute and Lead Investigator of the Diabetes Program at the Translational Research Institute. “This investigational therapy represents a potentially distinct asset in the field of weight management. The positive outcomes highlight the potential of this treatment and pave the way for further research and development.”
“The encouraging data from our GL0034 study underscore our dedication to combating the escalating global health crisis of obesity. These results not only show the effectiveness of GL0034 in achieving weight loss but also its promise in improving vital cardiometabolic parameters and risk factors,” said Dilip Shanghvi, Managing Director, Sun Pharma. “We are committed to advancing this innovative therapy to deliver meaningful benefits to patients around the world.”
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
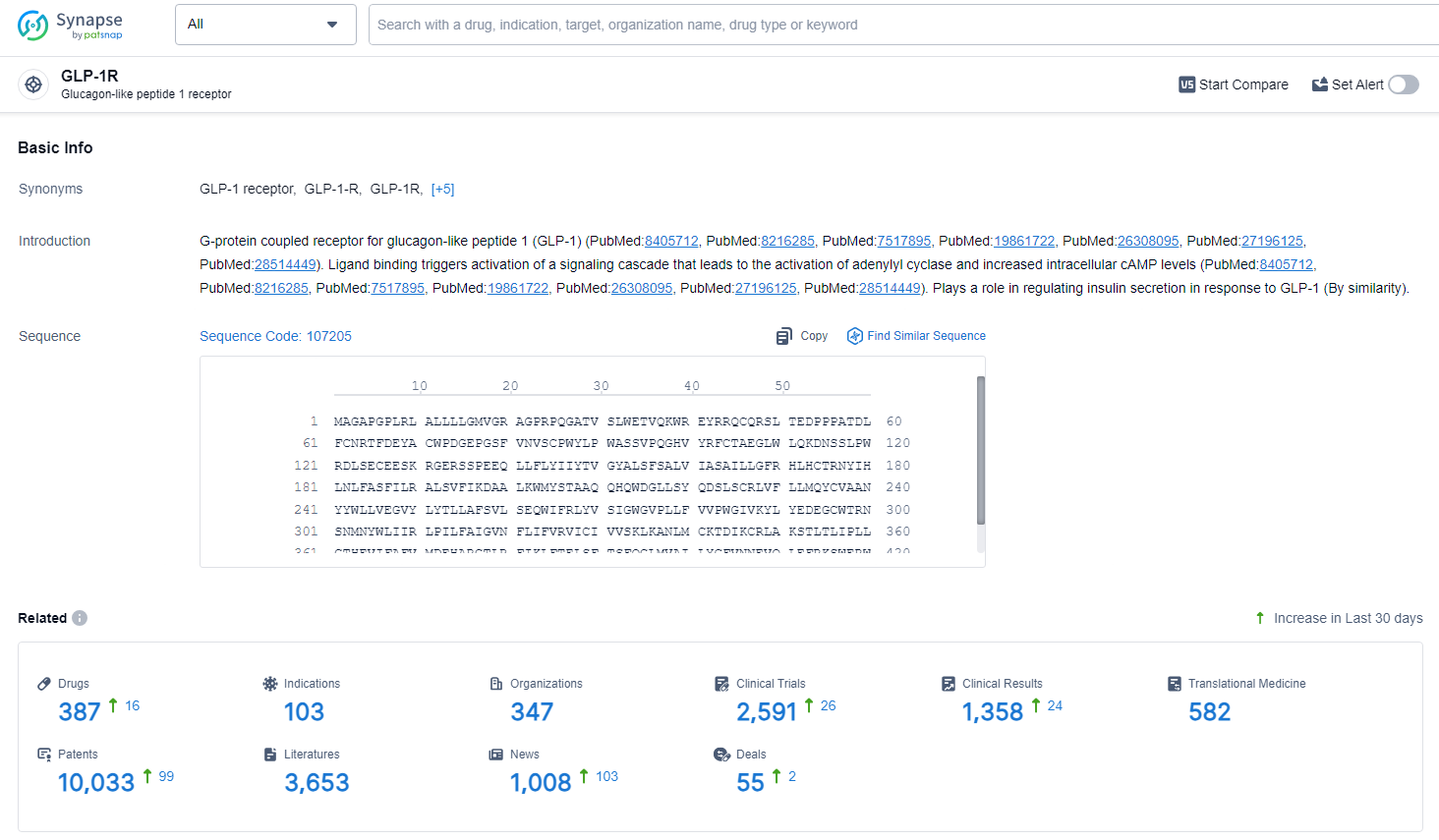
According to the data provided by the Synapse Database, As of June 27, 2024, there are 387 investigational drugs for the GLP-1R target, including 103 indications, 347 R&D institutions involved, with related clinical trials reaching 2591, and as many as 10033 patents.
Utreglutide targets the GLP-1R for the treatment of Diabetes Mellitus, Type 2, and Obesity. The drug has reached Phase 1 in its global development, indicating early-stage clinical testing. The potential of Utreglutide to address significant medical needs in the field of endocrinology and metabolic disease makes it a promising candidate for further development and evaluation.