Mineralys Therapeutics Announces First Patient Dosed in Start-HTN, Its Second Key Trial of Lorundrostat for Hypertension Control
Mineralys Therapeutics, Inc., an enterprise operating in the clinical phase and specializing in biopharmaceutical development, is geared towards crafting therapeutic solutions aimed at high blood pressure, persistent renal ailments, and various disorders caused by increased levels of aldosterone. The company has disclosed that initial dosing has commenced in their critical Launch-HTN trial. This study is designed to assess lorundrostat's reliability and effectiveness in addressing cases of hypertension that are not responsive to standard treatments, also known as resistant hypertension.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Starting the commencement of this critical second study signals the significant headway we've accomplished in advancing a precision medicine paradigm in the management of hypertension. This is done through the identification and categorization of individuals likely to benefit from lorundrostat treatment," announced Jon Congleton, the CEO of Mineralys.
Concurrently with our endeavors on lorundrostat, we have observed with satisfaction the growing body of evidence presented by the healthcare field that spotlights aldosterone as a principal factor in cardiorenal disorders," commented Jon Congleton further.
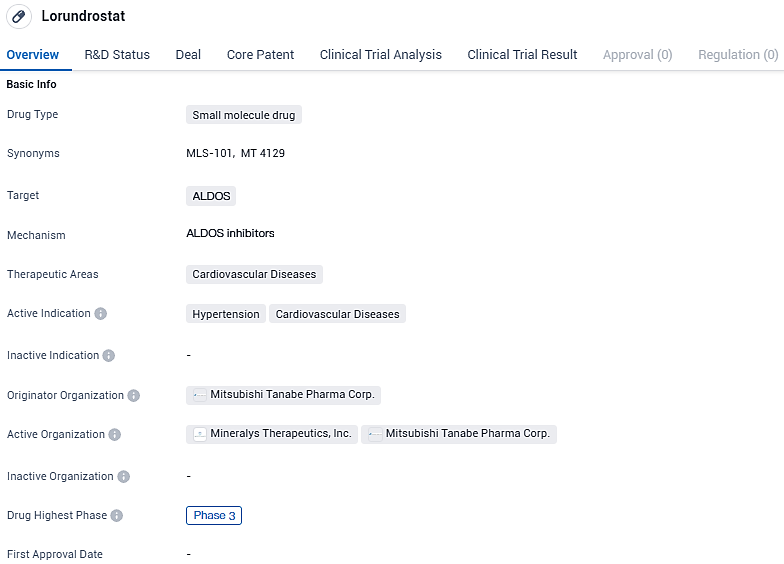
This clinical study aims to emulate the treatment patterns for uHTN and rHTN typically seen in general healthcare practices. The trial, known as Launch-HTN, is a worldwide, random assignment, dual-masked, placebo-supervised Phase 3 investigation. It plans to recruit close to 1,000 adult candidates who are not meeting their blood pressure targets while on a regimen of two to five antihypertensive treatments.
Qualified participants will be distributed randomly into one of three groups: a control placebo, a 50 mg daily dosage of lorundrostat, and a 50 mg daily dosage of lorundrostat with a potential increase to 100 mg daily after the initial six weeks if necessary. The principal measure for the trial's success is the variation from the starting systolic blood pressure compared to the placebo group, after a 12-week treatment period, as determined by automated office blood pressure evaluations.
Previously, as of May 2023, the entity proclaimed the initiation of dosing the pioneer participant in the first critical study, Advance-HTN.
Data from this Advance-HTN trial is anticipated to be publicly disclosed in the latter half of 2024. Participants engaged in both the Advance-HTN and Launch-HTN investigations are granted the choice to continue in the continuing open-label Transform-HTN trial.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
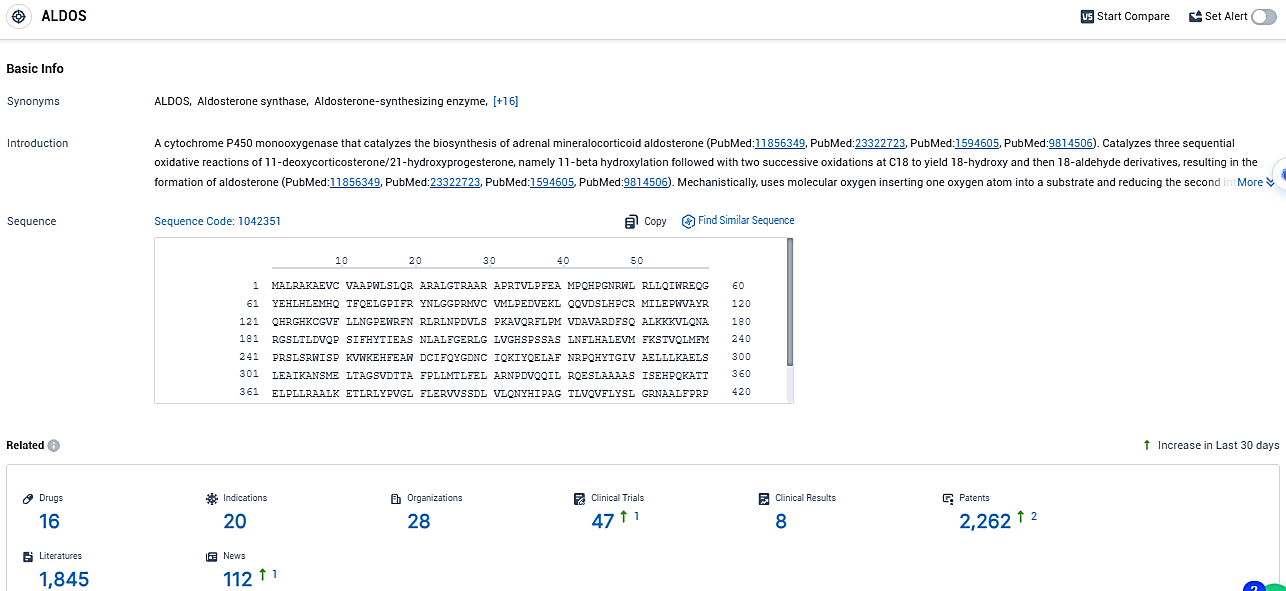
According to the data provided by the Synapse Database, As of December 30, 2023, there are 16 investigational drugs for the ALDOS target, including 20 indications, 28 R&D institutions involved, with related clinical trials reaching 47, and as many as 2262 patents.
Lorundrostat targets ALDOS and is primarily focused on treating hypertension and other cardiovascular diseases. The drug is currently in Phase 3 of clinical trials, indicating its advanced stage of development. With its potential to regulate blood pressure and improve cardiovascular health, Lorundrostat holds promise as a therapeutic option in the field of biomedicine.