moxifloxacin hydrochloride: Detailed Review of its Transformative R&D Success
moxifloxacin hydrochloride's R&D Progress
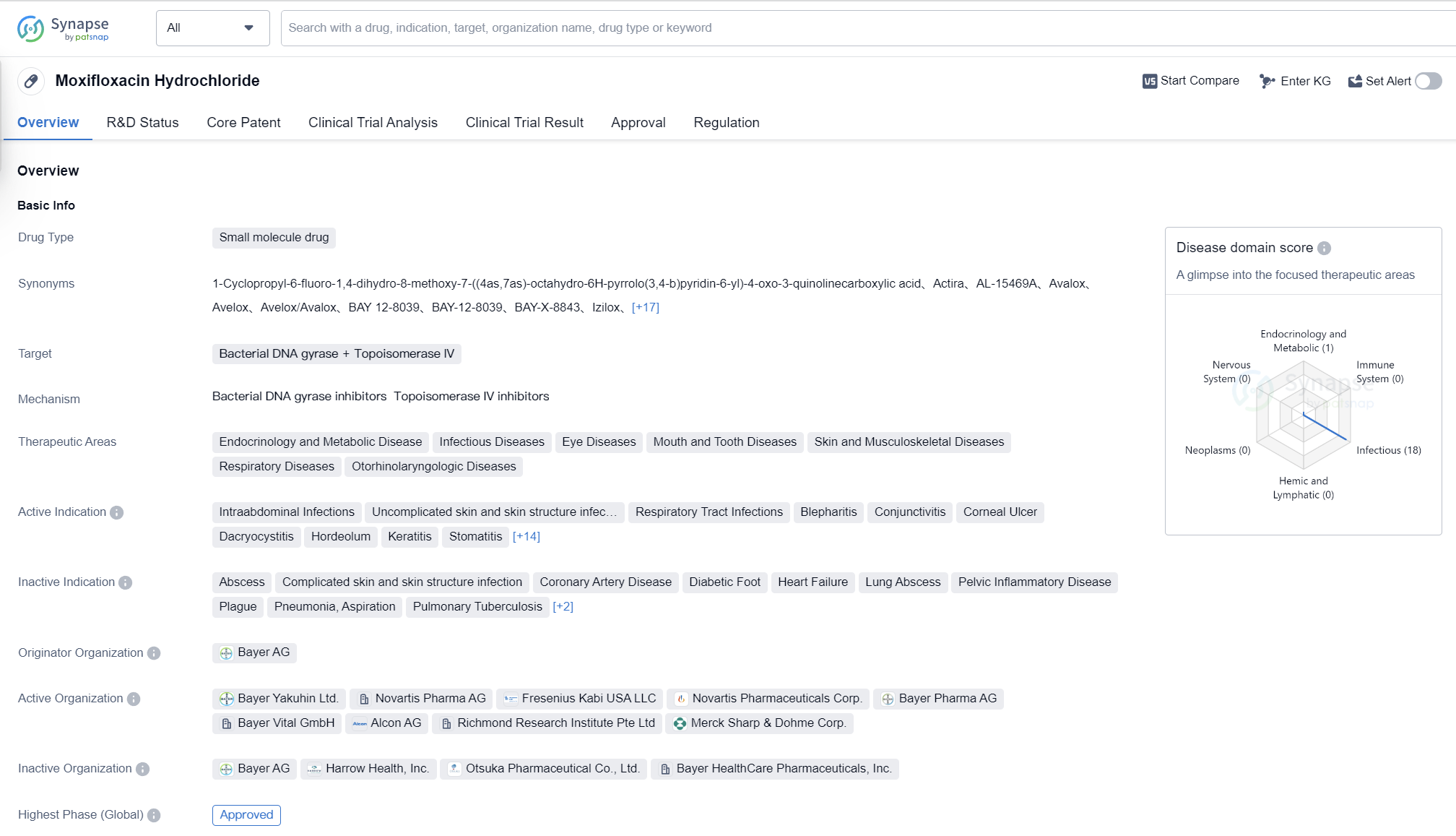
Moxifloxacin Hydrochloride is a small molecule drug that targets bacterial DNA gyrase and Topoisomerase IV. It has been approved for various therapeutic areas including endocrinology and metabolic disease, infectious diseases, eye diseases, mouth and tooth diseases, skin and musculoskeletal diseases, respiratory diseases, and otorhinolaryngologic diseases.
The drug is indicated for the treatment of intraabdominal infections, uncomplicated skin and skin structure infections, respiratory tract infections, blepharitis, conjunctivitis, corneal ulcer, dacryocystitis, hordeolum, keratitis, stomatitis, acute bronchitis, pharyngolaryngitis, pneumonia, respiratory tract infection (chronic), secondary infection, skin and skin structure infections, tonsillitis, conjunctivitis (bacterial), chronic bronchitis, community-acquired pneumonia, sinusitis, bacterial infections, endophthalmitis, and diabetes mellitus.
Moxifloxacin Hydrochloride was first approved in Germany in June 1999 by Bayer AG, the originator organization. It has also received approval in China. The drug has gone through the highest phase of clinical trials and has been approved for use globally.
Moxifloxacin Hydrochloride has undergone priority review and has been designated as an orphan drug, indicating that it has the potential to address an unmet medical need and is intended to treat rare diseases or conditions. Its expedited review by regulatory authorities demonstrates its importance in the medical field.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for moxifloxacin hydrochloride: Bacterial DNA gyrase inhibitors and topoisomerase IV inhibitors
Bacterial DNA gyrase inhibitors and topoisomerase IV inhibitors are types of drugs that target specific enzymes involved in DNA replication and repair in bacteria. These drugs are used to treat bacterial infections by interfering with the bacterial DNA replication process.
DNA gyrase is an enzyme that helps to relieve the torsional strain that occurs during DNA replication. It introduces negative supercoils into the DNA molecule, allowing it to unwind and separate into two strands. Topoisomerase IV is another enzyme that helps in the separation of replicated DNA strands during cell division.
Bacterial DNA gyrase inhibitors, as the name suggests, specifically target DNA gyrase. By inhibiting the activity of DNA gyrase, these drugs prevent the proper unwinding and separation of DNA strands, leading to the inhibition of bacterial DNA replication. This ultimately inhibits bacterial growth and helps in treating bacterial infections.
Topoisomerase IV inhibitors, on the other hand, target the enzyme topoisomerase IV. By inhibiting the activity of topoisomerase IV, these drugs prevent the separation of replicated DNA strands, leading to the formation of abnormal DNA structures. This disruption in DNA replication and segregation hinders bacterial cell division and growth, thereby aiding in the treatment of bacterial infections.
Overall, bacterial DNA gyrase inhibitors and topoisomerase IV inhibitors are important classes of drugs that specifically target bacterial enzymes involved in DNA replication and cell division. They play a crucial role in the treatment of bacterial infections by interfering with essential bacterial processes.
Drug Target R&D Trends for moxifloxacin hydrochloride
Bacterial DNA gyrase and topoisomerase IV are enzymes that play crucial roles in the replication and maintenance of bacterial DNA. DNA gyrase is responsible for introducing negative supercoils into the DNA molecule, which helps in the unwinding and separation of DNA strands during replication. Topoisomerase IV, on the other hand, is involved in the decatenation of daughter DNA molecules after replication. These enzymes are essential for bacterial survival and are targeted by certain antibiotics, such as fluoroquinolones, which inhibit their activity and disrupt bacterial DNA replication, ultimately leading to bacterial cell death.
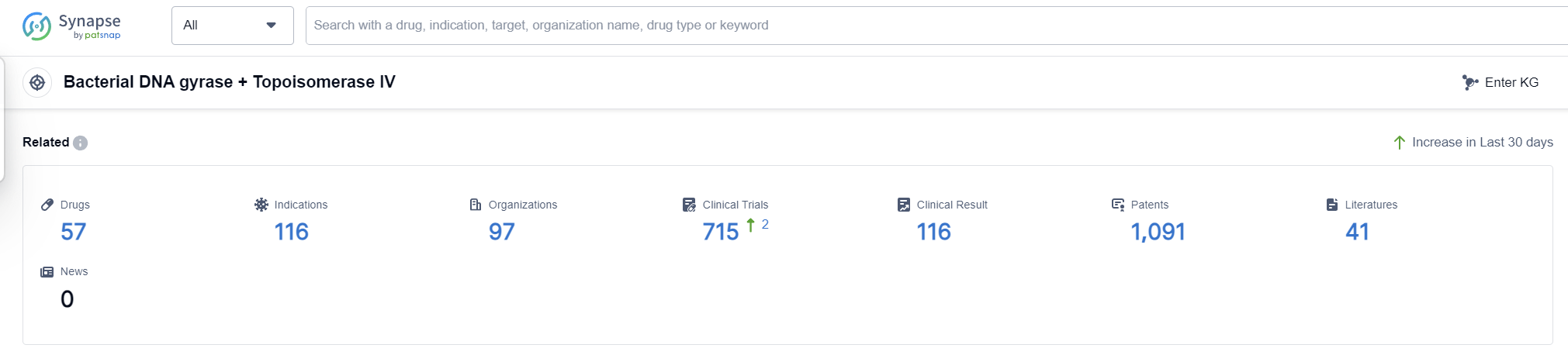
According to Patsnap Synapse, as of 6 Sep 2023, there are a total of 57 Bacterial DNA gyrase + Topoisomerase IV drugs worldwide, from 97 organizations, covering 116 indications, and conducting 715 clinical trials.
Overall, the target Bacterial DNA gyrase + Topoisomerase IV presents a competitive landscape with multiple companies and countries actively involved in the development of drugs. The future development of this target is promising, with ongoing research and development efforts focused on addressing various indications related to bacterial infections.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Moxifloxacin Hydrochloride is a small molecule drug that targets bacterial DNA gyrase and Topoisomerase IV. It has been approved for various therapeutic areas and is indicated for the treatment of a wide range of infections and diseases. The drug was first approved in Germany in 1999 and has since received approval in China. It has undergone priority review and has been designated as an orphan drug.