Nektar Launches Phase 2b Trial for Severe Alopecia Areata Treatment
Nektar Therapeutics, an enterprise specializing in the creation of drugs to combat autoimmune conditions, has declared the commencement of its advanced Phase 2b medical study to assess the efficacy of rezpegaldesleukin in individuals suffering from extreme cases of alopecia areata.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
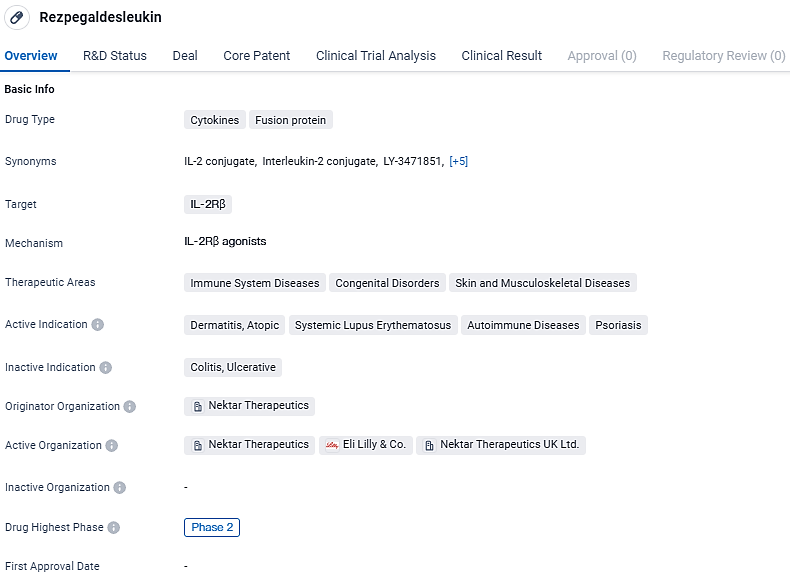
Rezpegaldesleukin represents an innovative therapeutic approach, functioning as an agonist for T regulatory cells, with the dual purpose of mitigating inflammation and reinstating equilibrium within the immune system. This is achieved through direct amplification of active T regulatory cells and interaction with a variety of immune regulatory mechanisms.
Commenting on the progression of the Phase 2b trial, Nektar's Chief Medical Officer, Dr. Mary Tagliaferri, expressed the significant progress this represents for the company, highlighting the promise of REZPEG as an entirely new therapeutic approach for not just alopecia areata but also other autoimmune conditions. Dr. Tagliaferri expressed optimism about REZPEG's potential to offer a groundbreaking therapeutic pathway for patients with alopecia and is looking forward to initial data from the trial, which is expected in early 2025.
Nektar is embarking on a comprehensive Phase 2b trial on a worldwide scale. This study is methodically structured as a double-blind, placebo-controlled trial to assess the REZPEG effectiveness and safety profile across a participant group of 84 individuals diagnosed with severe alopecia areata. This will take place over a 36-week period of induction therapy. The study will contrast REZPEG given at two different doses against a placebo. To assess long-term effects, a 24-week monitoring period will follow post-treatment. The initial findings from this research are projected to become available in the early part of 2025.
The key measure for treatment success is the average percentage increase in the Severity of Alopecia Tool (SALT) scores by the 36th week. Other secondary measures include the proportion of subjects achieving a 50% or greater reduction in their SALT score by week 36 and at other predefined timepoints, along with the average percentage increase in SALT scores at these various timepoints.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
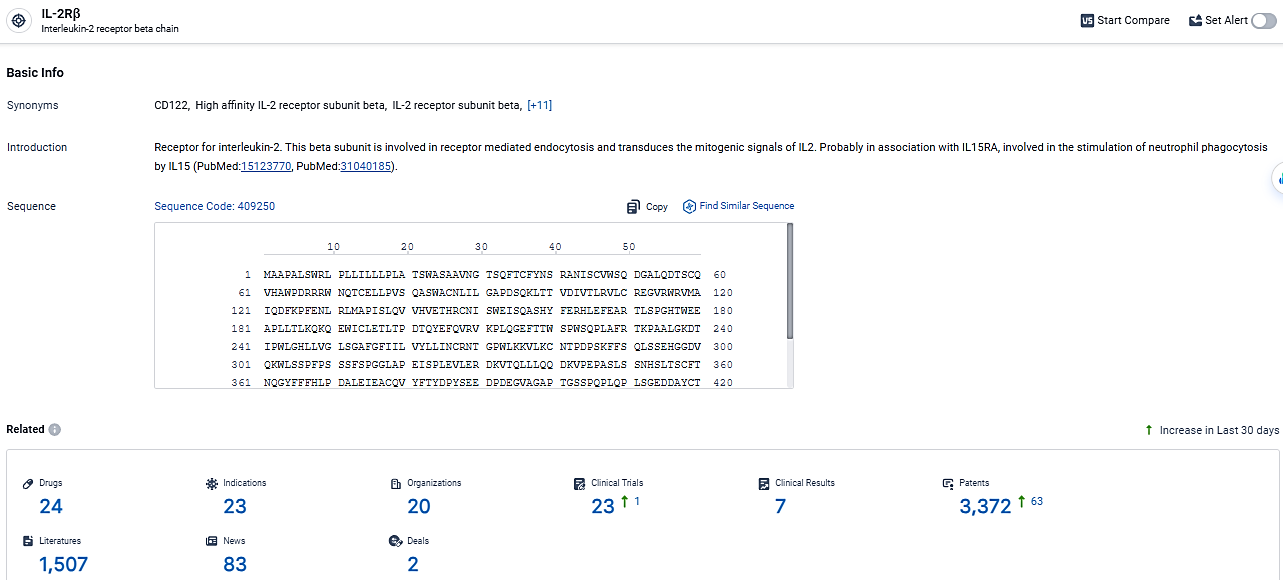
According to the data provided by the Synapse Database, As of March 6, 2024, there are 24 investigational drugs for the IL-2Rβ target, including 23 indications,20 R&D institutions involved, with related clinical trials reaching 23, and as many as 3372 patents.
Rezpegaldesleukin is a cytokine fusion protein that targets IL-2Rβ and is being developed by Nektar Therapeutics. It has shown potential for the treatment of immune system diseases, congenital disorders, and skin and musculoskeletal diseases. The drug has reached Phase 2 of clinical development globally and has reached the stage of IND application in China. Further clinical trials will be conducted to assess its safety and efficacy in treating the indicated conditions.