Nektar Therapeutics Unveils Initial Preclinical Data for CSF-1 Program NKTR-422 at 2024 ACR Convergence
Nektar Therapeutics (Nasdaq: NKTR) has announced that it will present an oral session featuring preclinical findings on NKTR-422 during the 2024 American College of Rheumatology (ACR) meeting, which is set to take place in Washington, D.C. from November 14 to 19, 2024.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
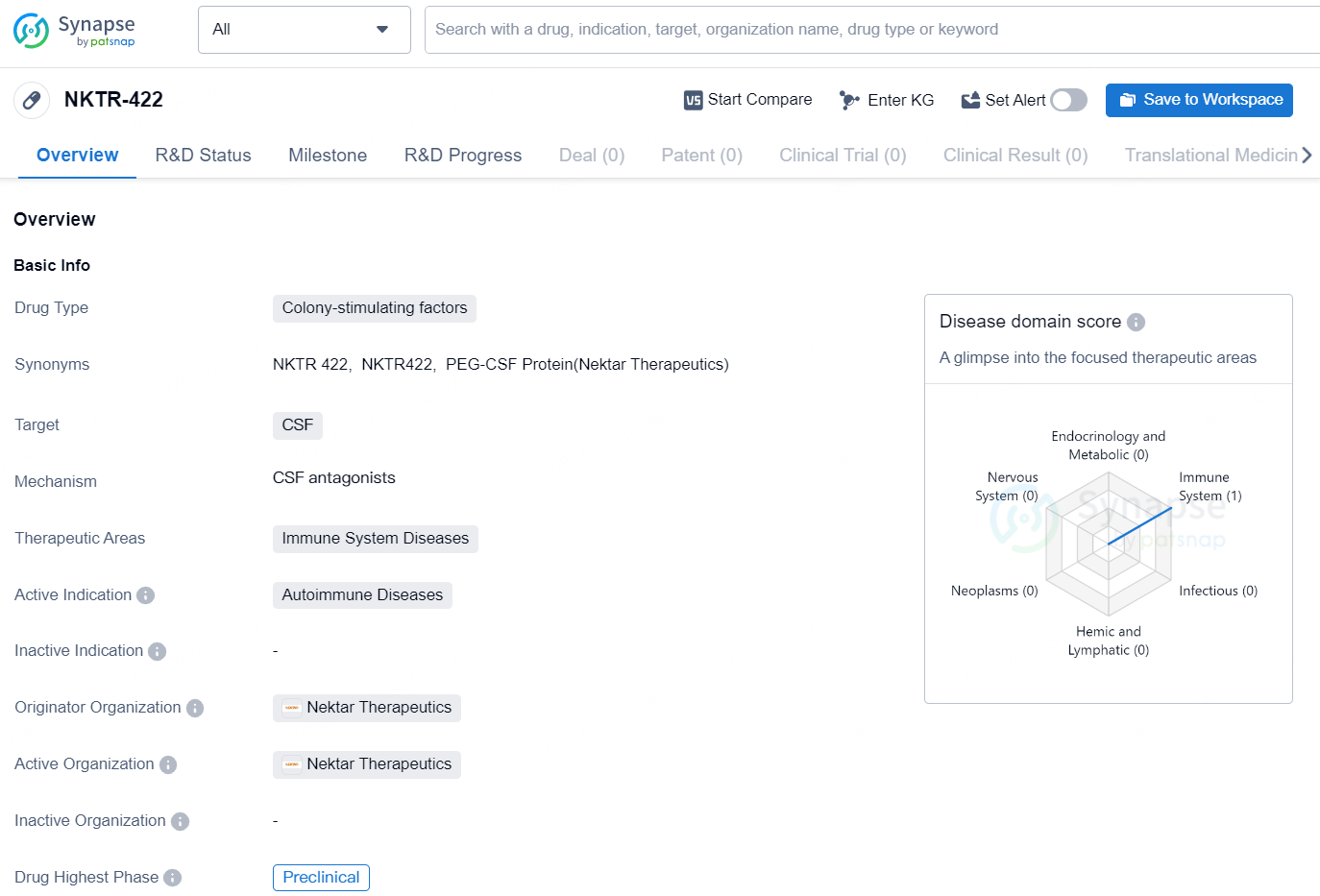
NKTR-422 is an innovative modified hematopoietic colony stimulating factor (CSF) protein designed to selectively influence the resolution of inflammation. It does this by targeting the proliferation, reprogramming, and activation of anti-inflammatory tissue resident macrophages.
Current therapies for inflammatory diseases do not focus on promoting inflammation resolution or facilitating tissue repair, both of which are essential for achieving remission.1 Nektar has discovered a CSF-1R agonist that exhibits a unique pharmacokinetic (PK) and pharmacodynamic (PD) profile compared to the natural CSF-1 cytokine. Its reduced clearance allows for prolonged PD activity following a single administration, contrasting with the traditional requirement for multiple daily doses of recombinant human CSF-1. NKTR-422 has shown the ability to induce markers related to inflammation resolution and tissue repair in tissue resident macrophages, without causing monocytosis, and has improved the effectiveness of inflammatory cytokine blockade in rodent models.
“Preliminary findings suggest that NKTR-422 holds promise for enhancing treatment effectiveness and could promote disease remission, particularly when used in combination with established inflammatory cytokine blockade therapies,” stated Jonathan Zalevsky, Ph.D., Senior Vice President and Chief Research & Development Officer at Nektar. “We are eager to observe the progression of this program, given its broad potential applications in various therapeutic areas, including both acute and chronic inflammation.”
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
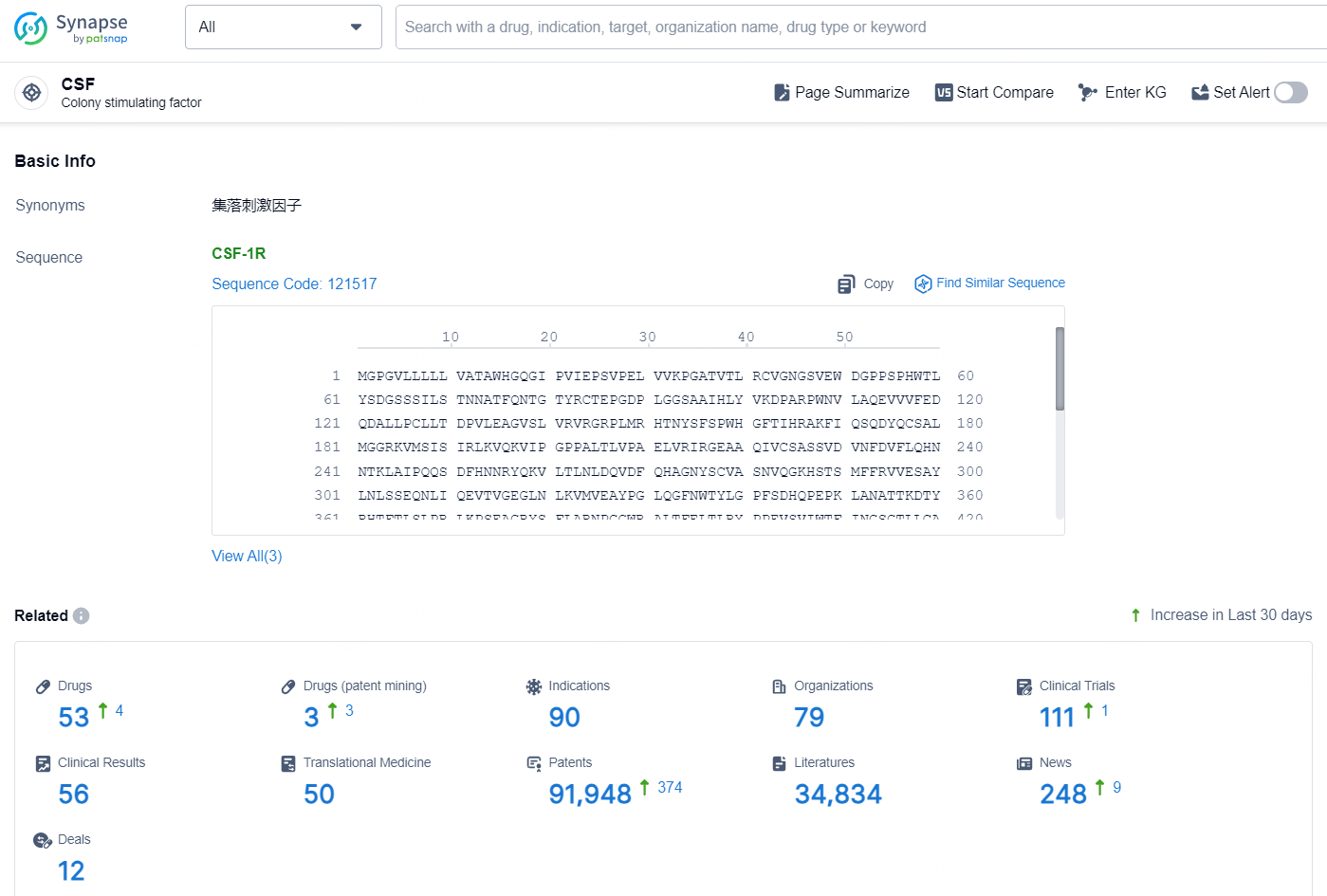
According to the data provided by the Synapse Database, As of November 25, 2024, there are 53 investigational drugs for the CSF target, including 90 indications, 79 R&D institutions involved, with related clinical trials reaching 111, and as many as 91948 patents.
NKTR-422 is a colony-stimulating factor drug developed by Nektar Therapeutics. It targets colony-stimulating factors (CSF) and is intended for the treatment of autoimmune diseases within the therapeutic area of immune system diseases. As of now, NKTR-422 is in the highest global phase of preclinical development.