Neurocrine's VMAT2 inhibitor Valbenazine achieves endpoint in Phase 3 clinical trial
Recently, Neurocrine Biosciences announced new data for the VMAT2 inhibitor drug Ingrezza (valbenazine) capsules, including preliminary results from the Phase III KINECT-HD clinical trial. The findings show that from week 2 to week 12, Ingrezza displayed a consistent and superior improvement in treating the chorea associated with Huntington's disease (HD) compared to the placebo.
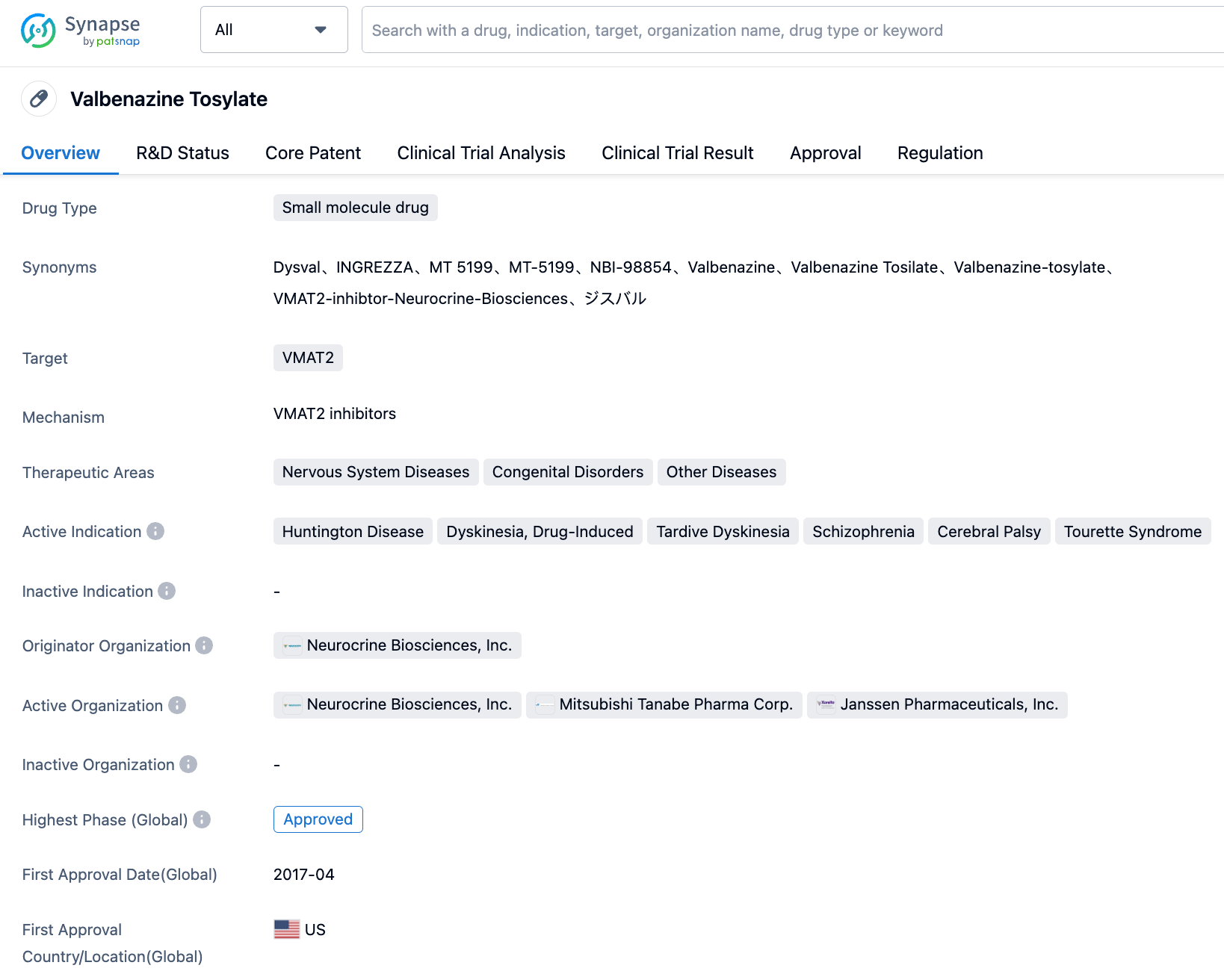
Valbenazine is a small molecule inhibitor targeting vesicular monoamine transporter 2 (VMAT2) that alleviates chorea symptoms and motor disorders by reducing pre-synaptic dopamine levels. By selectively targeting VMAT2, Valbenazine is thought to reduce surplus dopamine signaling, which could consequently decrease uncontrollable movements. On August 18th of this year, Valbenazine was approved by the FDA for the treatment of chorea symptoms in adult patients with Huntington's disease.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
KINECT-HD is a randomized, double-blind, placebo-controlled study that evaluated the efficacy and safety of Ingrezza. Previously published pivotal clinical trial results revealed that Ingrezza improved the severity of Huntington's disease (HD) chorea to three times that of a placebo, with an improvement of 4.6 points in the Ingrezza group's HD chorea severity rating from the start of the clinical trial to the 12th week, compared to an improvement of 1.4 points in the placebo group (95%CI, -4.4, -2.0; P < 0.0001). At the 12th week, Ingrezza reduced the severity of HD chorea from baseline in nearly half the patients by approximately 40% (P < 0.0001). Also at the 12th week, 53% of patients and 43% of healthcare professionals reported a "very great improvement" or "marked improvement" in overall HD chorea symptoms.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
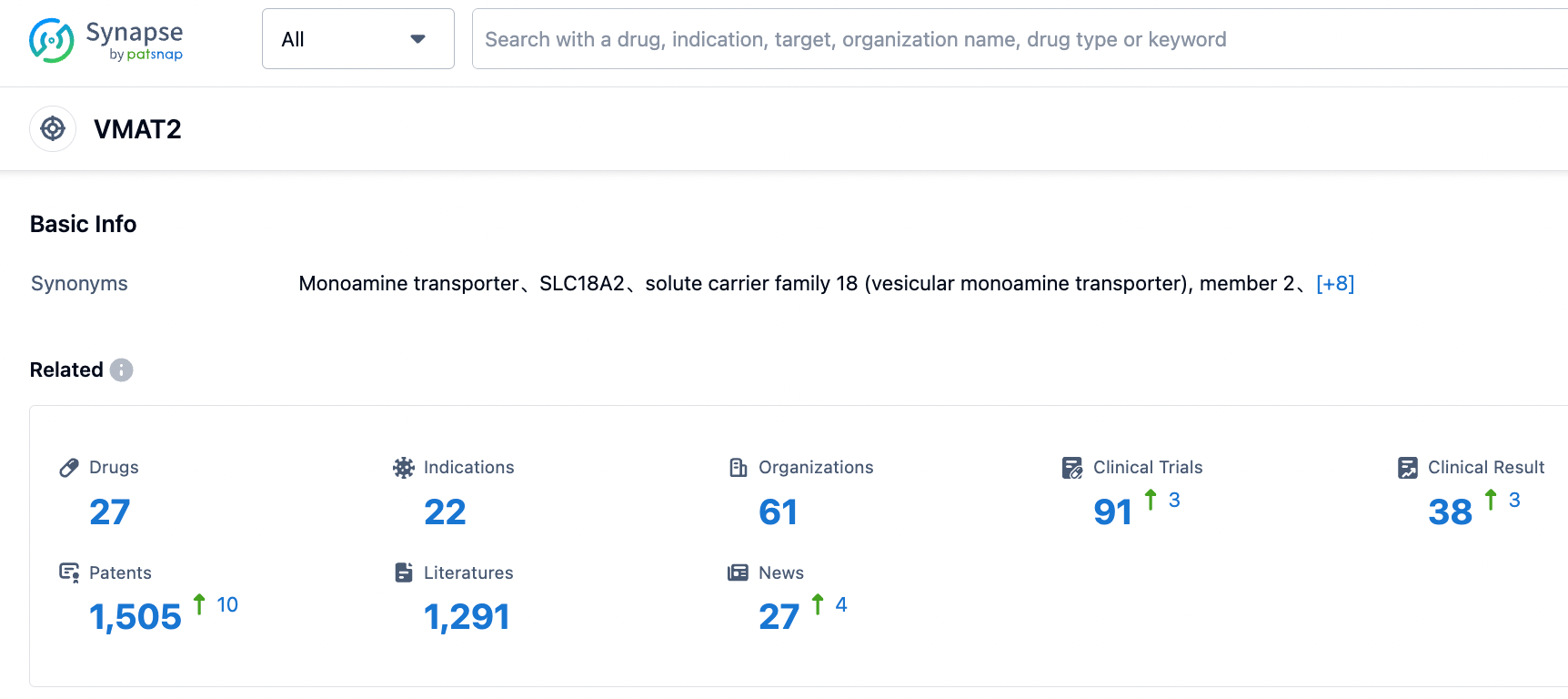
According to the information disclosed by the synapse database, as of September 1, 2023, there are a total of 27 drugs under investigation for the VMAT2 target, covering 22 indications, with 61 research institutions involved, 91 related clinical trials, and as many as 1512 patents... Previously, only three drugs were approved worldwide for the treatment of Huntington's disease, namely Tetrabenazine, Deutetrabenazine and Sulpiride. The approval of Valbenazine brings a new treatment option for patients with Huntington's disease.