The Phase 1 clinical trial for the KRAS G12D inhibitor MRTX1133, developed by Mirati, has been launched
Recently, researchers from MD Anderson Cancer Center published two studies in the sub-journals Developmental Cell and Cancer Cell, revealing not only the role of KRAS mutations in pancreatic cancer but also demonstrating that in preclinical models, the combined therapy of KRAS G12D inhibitor MRTX1133 and immune checkpoint inhibitors can cause sustained tumor elimination and significantly improve the survival outcomes in mice.
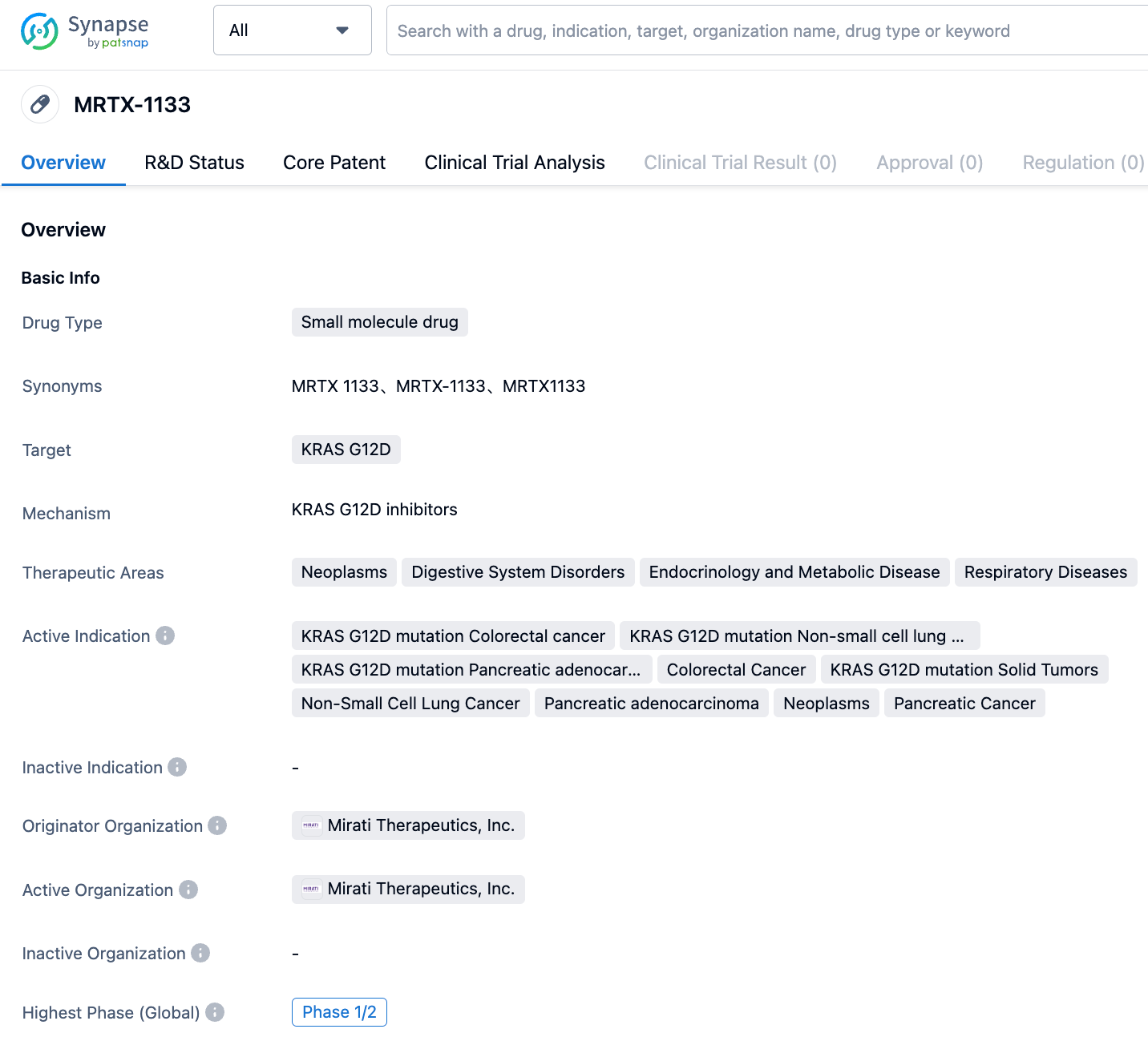
MRTX1133 is a selective non-covalent KRAS G12D inhibitor developed by Mirati Therapeutics. The related Phase 1 clinical trial (NCT05737706) has been launched, and patient recruitment is currently underway. In vitro models have shown that MRTX1133 has high inhibitory activity and powerful anticancer effects. In various pancreatic cancer cell transplantation models, MRTX1133 not only has effective monotherapy activity but also shows potential trends in combination therapy. KRAS mutations include not only G12C but also G12D and G12V, studies showed that 45% of global colorectal and pancreatic cancer patients and 17% of NSCLC patients carry KRAS G12D mutations. Therefore, drugs targeting KRAS G12D have a huge market potential.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
In this study published in Developmental Cell, the authors used several genetically engineered mouse models of pancreatic cancer with different KRAS mutations. They found that conditionally deleting KRAS in these mice activated the Fas pathway required for cancer cell death, leading to an increased number of T cells and a decrease in myeloid cells in the tumors.
Inhibition of KRAS also resulted in complete tumor regression and significant improvement in survival, suggesting that KRAS inhibition may improve the patient's response to immunotherapy and prevent recurrence. Analysis showed that tumor elimination depended on the activation of CD8+ T cells. If CD8+ T cells were inhibited, tumors would still progress despite treatment with MRTX1133.
Notably, when the researchers combined various immune checkpoint inhibitors with MRTX1133 in mice, it led to sustained tumor regression, enhanced cancer cell clearance, and improved survival outcomes. These experimental results were published in the journal Cancer Cell.
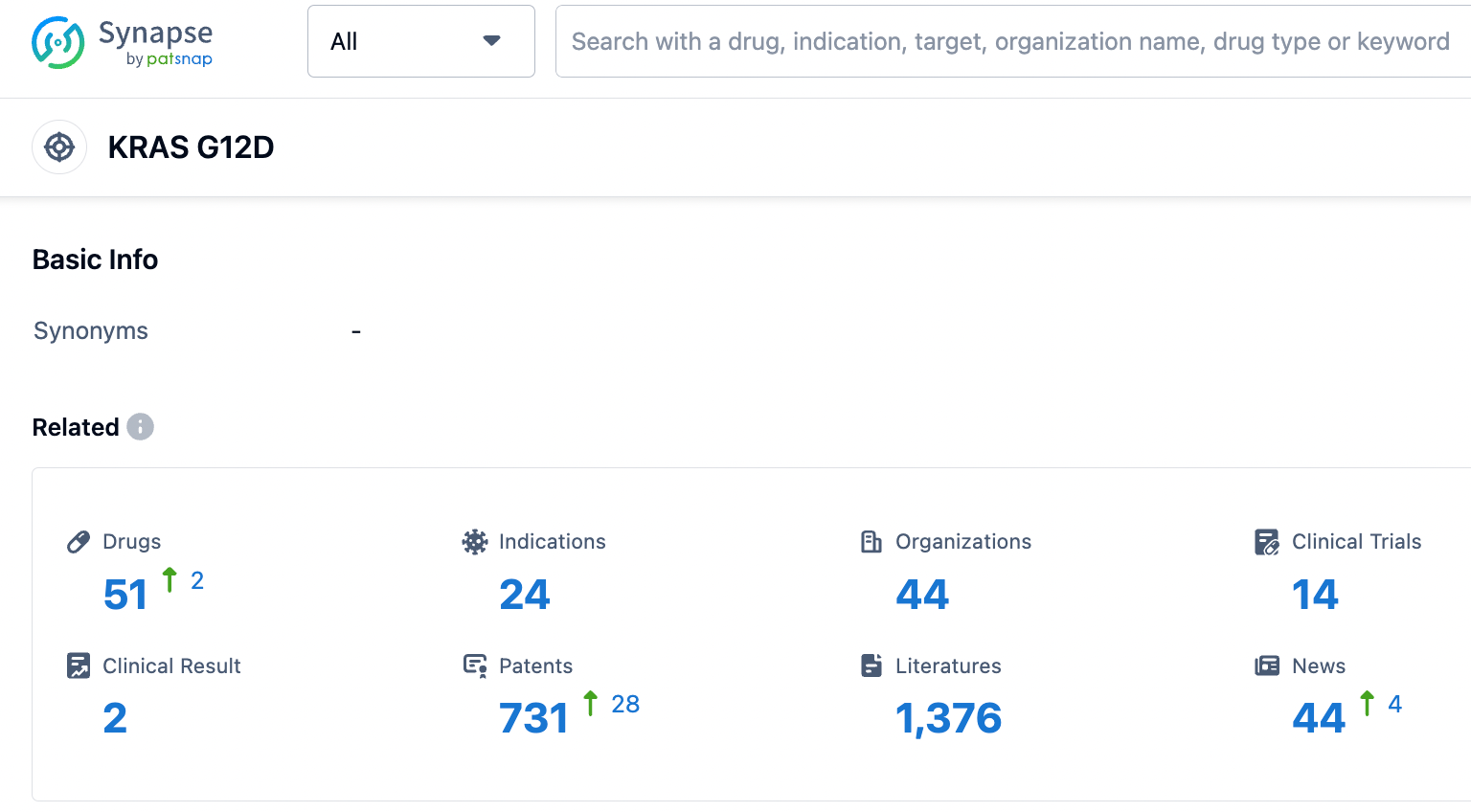
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the information disclosed by the Synapse database, as of August 31, 2023, there are a total of 51 drugs under development that target KRAS G12D, covering 24 different indications, with 44 institutions working on this research, 14 related clinical trials involved, and as many as 731 patents.
KRAS G12D is a potential target for designing mutation-selective KRAS inhibitors, with a mutation frequency three times higher in human cancers than KRAS G12C. MRTX1133 offers an opportunity to explore the role of the carcinogenic KRAS G12D mutation in the pathogenesis and progression of various types of cancer, and we look forward to its subsequent performance.