NextPoint Therapeutics Begins Clinical Trial for NPX887 Targeting HHLA2+ Solid Tumors
NextPoint Therapeutics has divulged the initiation of treatment in their first human subject within a Phase 1 clinical trial, using their investigational agent NPX887. This trial targets the therapeutic intervention for individuals carrying tumors marked by the presence of HHLA2/B7-H7, which is a noteworthy tumor-associated antigen, commonly found to be significantly overexpressed in a variety of human malignancies, irrespective of PD-L1 status.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
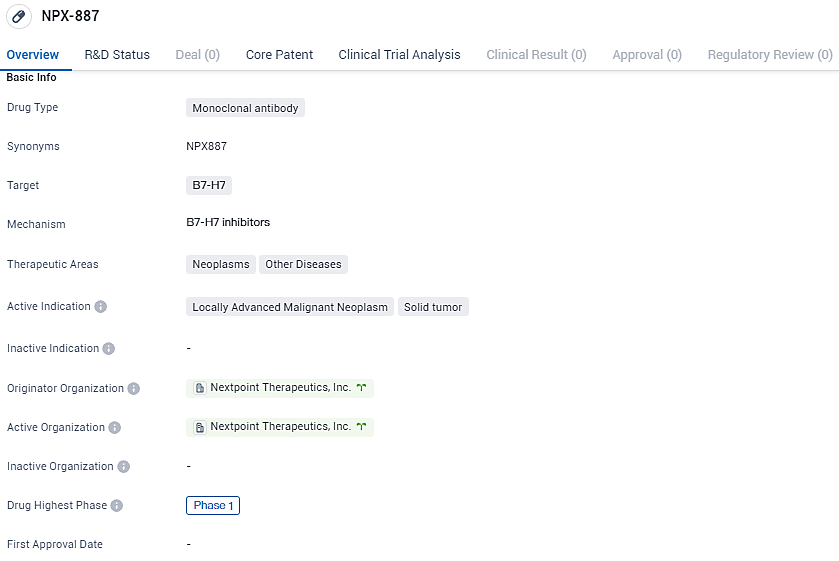
The research encompasses a Phase 1a/1b non-blinded, multi-institutional study which is composed of a period for assessing dose increment and a broader phase for examining the safety profile, pharmacodynamics, immune response elicitation, and biomarker-directed selection for NPX887 in patients affected by various solid tumor conditions. These include but are not limited to non-small cell lung cancer, renal cell carcinoma, colorectal cancer, and additional solid tumor categories recognized for HHLA2/B7-H7 expression.
Regarding the initiation of NPX887, our pioneering therapy aimed at the HHLA2/B7-H7 pathway, Leena Gandhi, MD, PhD, Chief Medical Officer at NextPoint Therapeutics, noted the significance in expanding our therapeutic approach geared toward re-engaging the immune combat against cancer with this innovative route.
Dr. Gandhi also highlighted, "With both NPX267 and NPX887 in development—our initial and secondary clinical ventures focused on targeting the KIR3DL3 receptor and HHLA2, respectively—we are at the forefront of examining the optimal use of this mechanism. This is specifically for treating those with tumors exhibiting HHLA2, presenting a distinct tumor-immune checkpoint divergent from PD-L1. Our aspiration for these clinical pursuits is hinged on harnessing HHLA2 as a strategic biomarker to refine the selection process for patient populations apt to experience therapeutic gains. Both agents are anticipated to deliver singular therapeutic advantages across certain demographics of patients."
The therapeutic agent NPX887 is designed as a fully humanized monoclonal antibody centered on HHLA2 (B7-H7), an emerging immune checkpoint and antigen that's prevalent in a multitude of cancers, manifesting independently of PD-L1. The mode of action implicated with NPX887 involves inhibiting the immunosuppression mediated by KIR3DL3 interactions with HHLA2. It is this inhibition which is posited to bolster the antitumor responses from T cells and NK cells within the tumor milieu.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
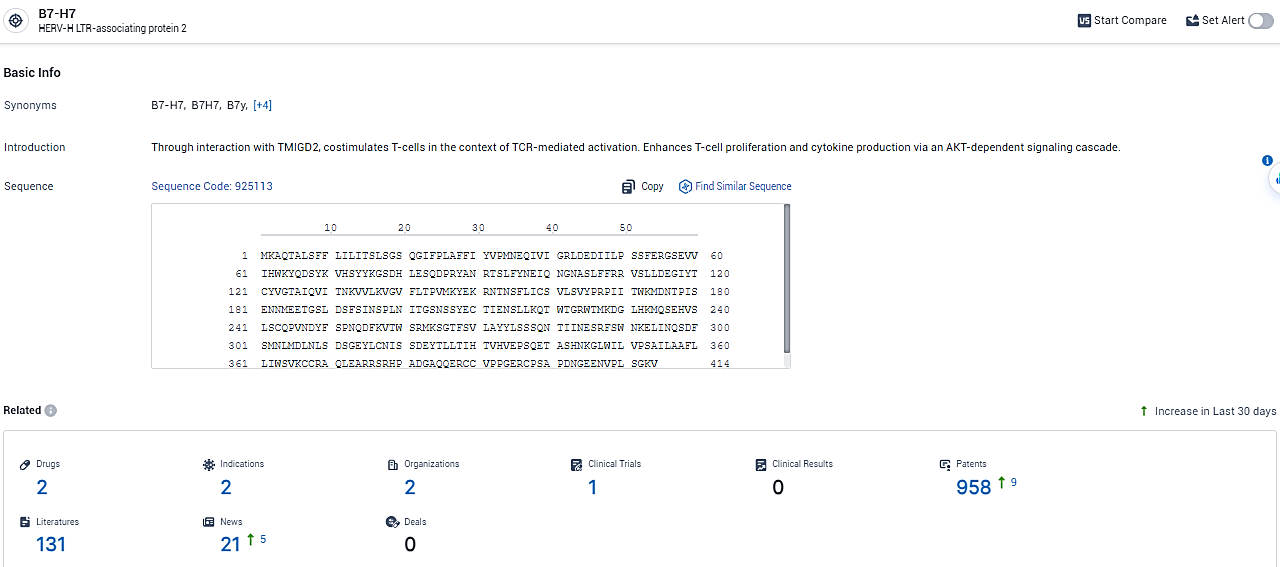
According to the data provided by the Synapse Database, As of February 26, 2024, there are 2 investigational drugs for the B7-H7 target, including 2 indications,2 R&D institutions involved, with related clinical trials reaching 1, and as many as 958 patents.
NPX-887 targets B7-H7 and is primarily intended for the treatment of locally advanced malignant neoplasms and solid tumors. The drug is currently in Phase 1 of clinical development, and its therapeutic areas include neoplasms and other diseases.