Nitroxoline: A Quick Look at Its R&D Progress and Clinical Results from the 2024 ASCO_GU
APL-1202 (nitroxoline) is a reversible and orally available MetAP2 inhibitor with anti-angiogenic and anti-tumor activities. Single-agent neoadjuvant programmed death 1 (PD-1) antibodies achieve pathological complete responses in a subset of patients (pts) with muscle-invasive bladder cancer (MIBC). APL-1202 and PD-1 antibody combination therapy demonstrates synergistic effects in several model systems of cancer, including bladder cancer. Recently, the clinical results of Nitroxoline, in combination with tislelizumab, a humanized IgG4 anti-PD-1 antibody, were presented in 2024 ASCO_GU, demonstrating an effective neoadjuvant therapy in MIBC.
Nitroxoline's R&D Progress
Nitroxoline is a small molecule drug that is being developed to target METAP2. It is primarily being investigated for its potential therapeutic applications in neoplasms and urogenital diseases. The active indications for Nitroxoline include non-muscle invasive bladder neoplasms, bladder cancer, and muscle invasive bladder carcinoma.
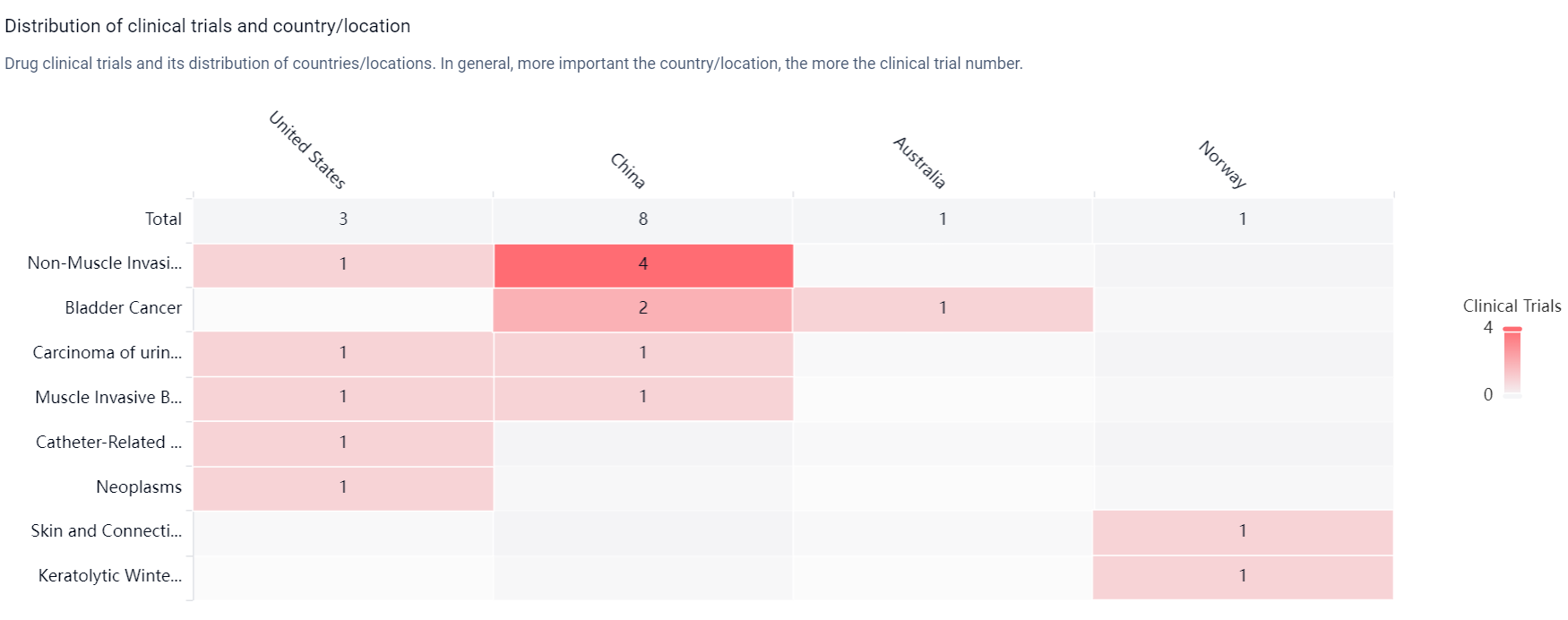
According to the Patsnap Synapse, Nitroxolin has reached the highest phase of clinical development, which is Phase 3 globally. And the clinical trial distributions for Nitroxoline are primarily in the United States, China and Australia. The key indication is Non-Muscle Invasive Bladder Neoplasms.
Detailed Clinical Result of Nitroxoline
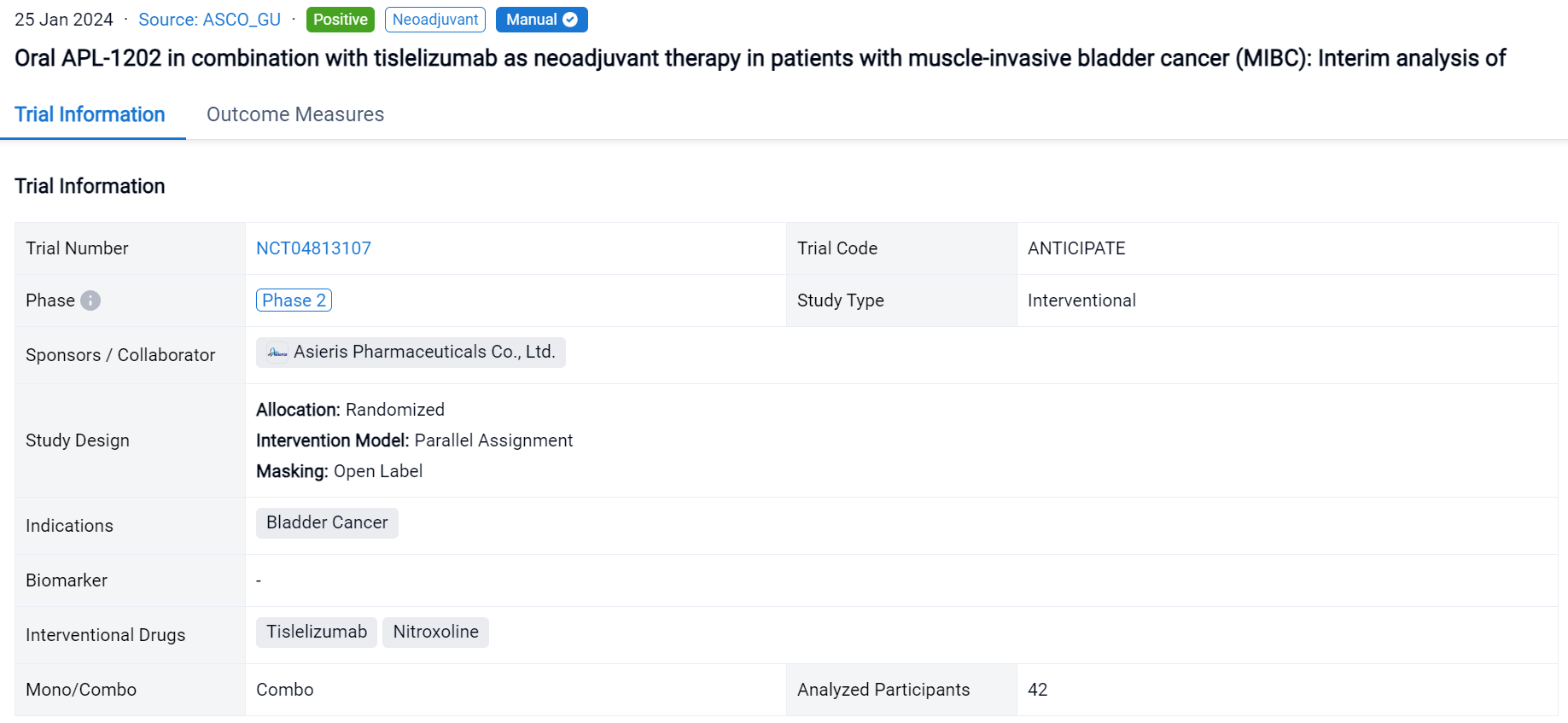
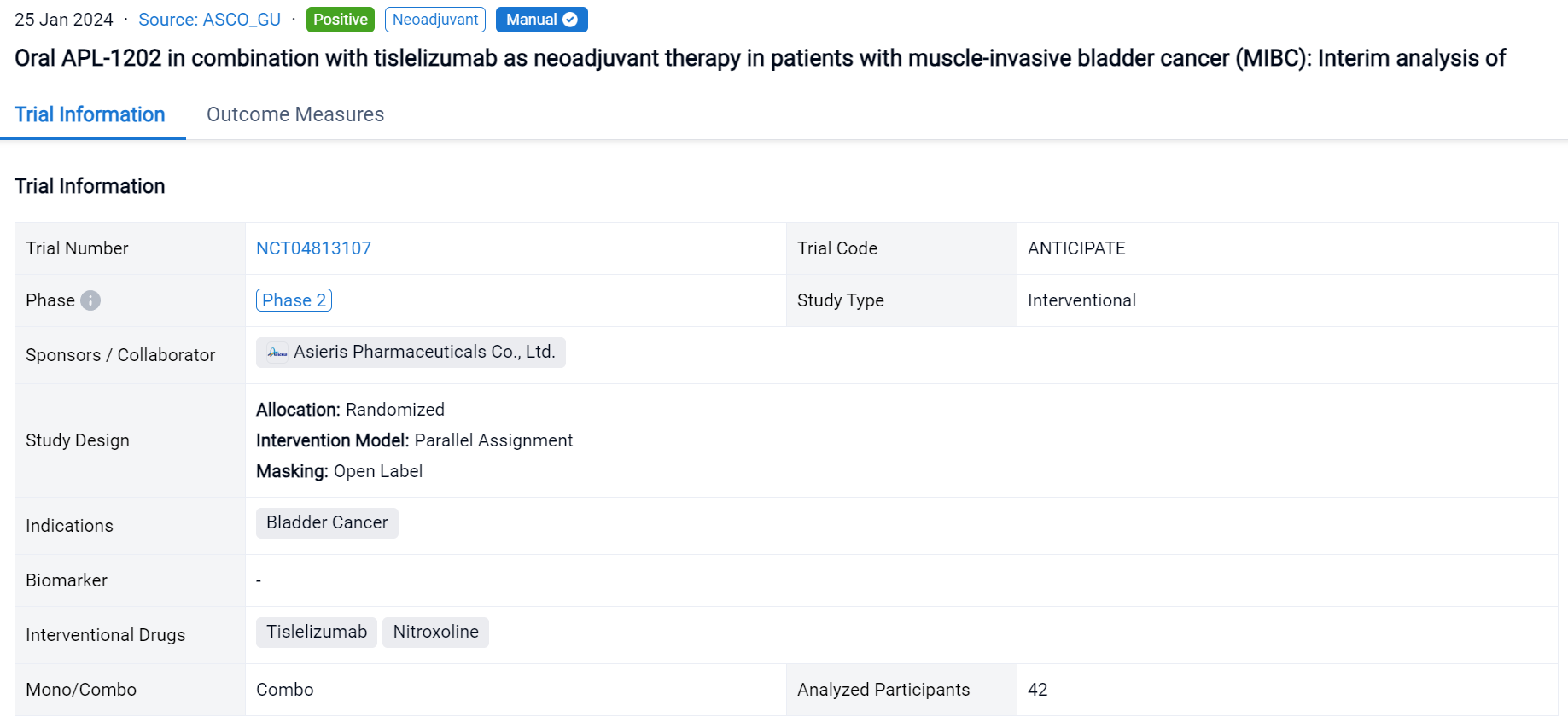
This randomized, parallel assignment, open-labeled clinical trial (NCT04813107) was aimed to assess the efficacy and safety of APL-1202 plus tislelizumab as an effective neoadjuvant therapy in MIBC.
In this study, eligible pts are randomly assigned to group 1 (APL-1202 plus tislelizumab) or group 2 (tislelizumab), with randomization stratified by PD-L1 expression. Neoadjuvant treatment is administered every 3 weeks for 3 cycles. The primary endpoint is pathological complete response (pCR, pT0N0) rate. A Simon’s 2-stage design is employed with planned interim analyses after 18 evaluable pts in group 1 and 14 evaluable pts in group 2.
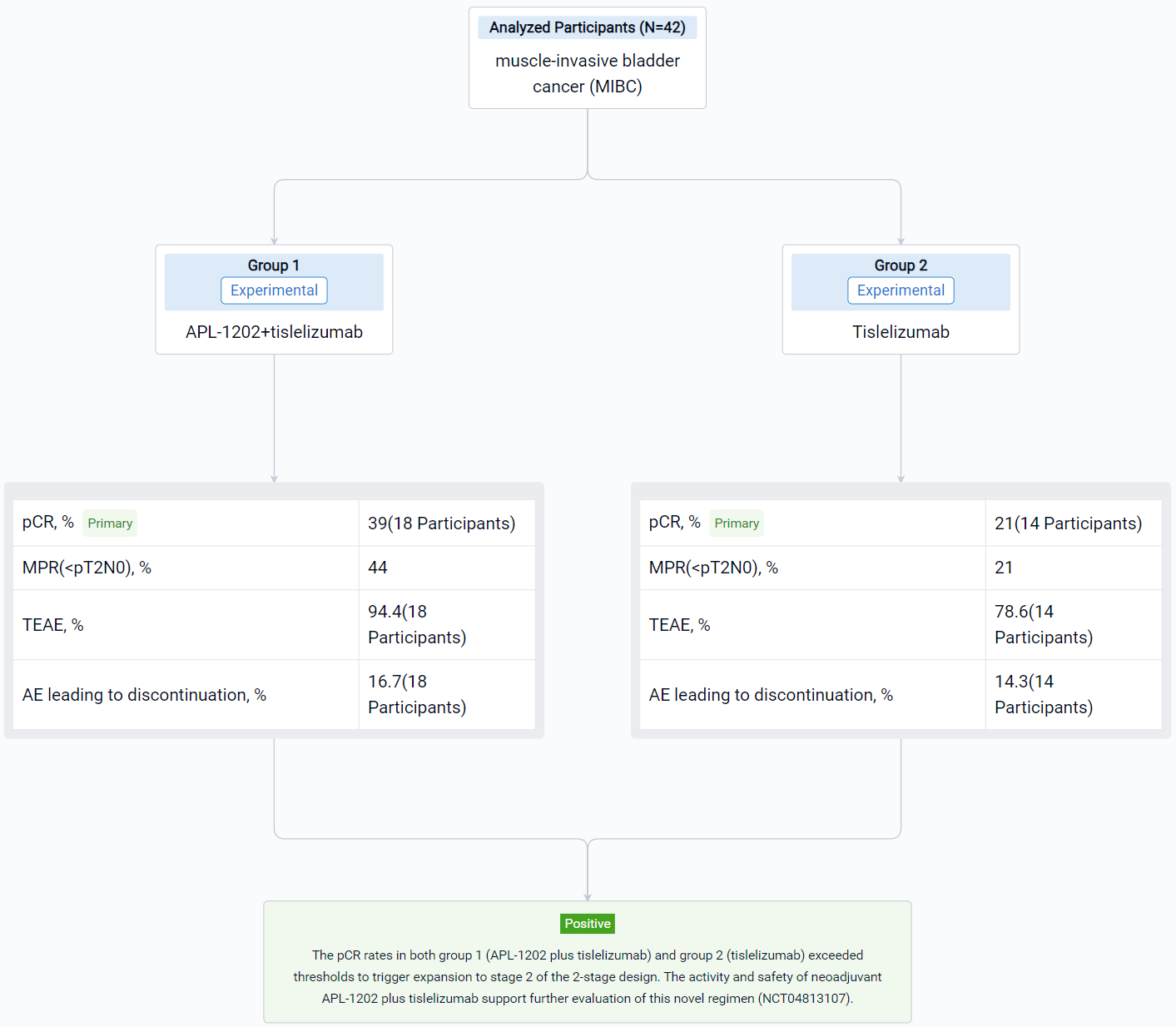
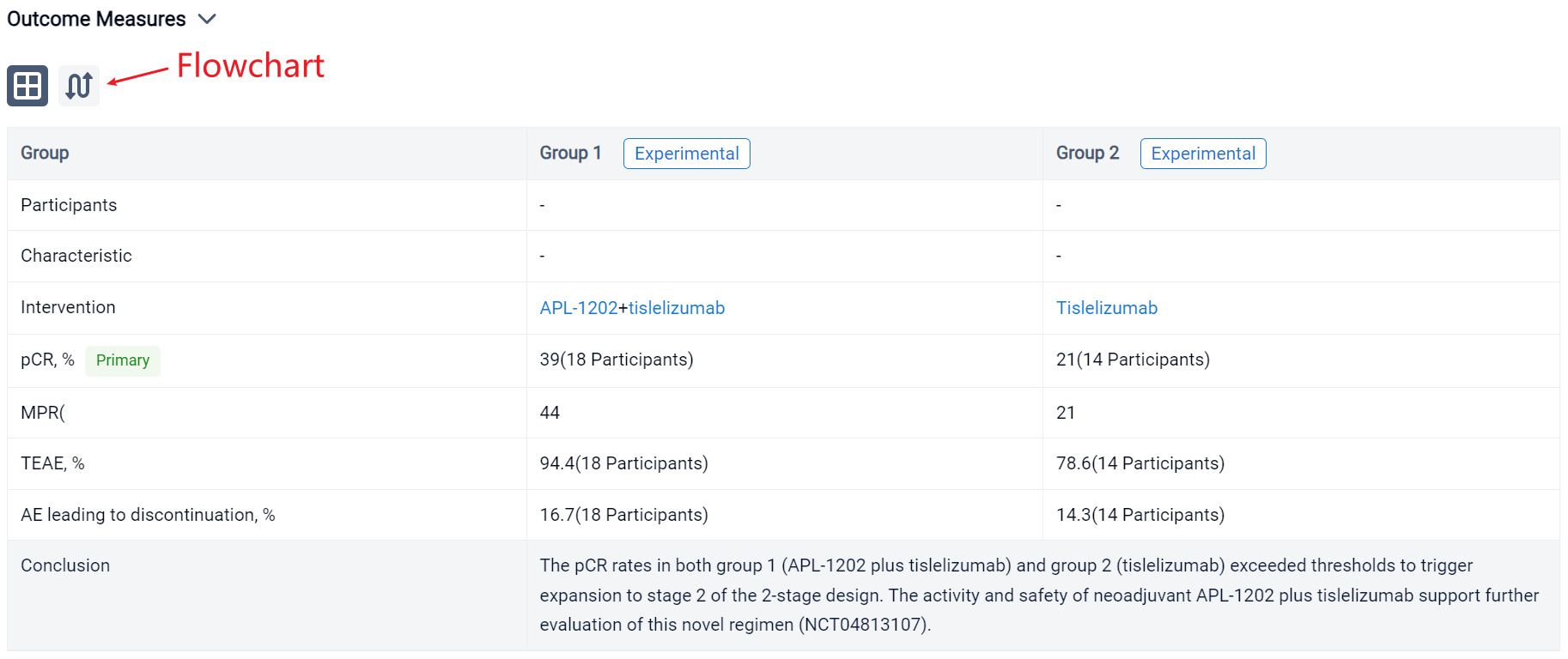
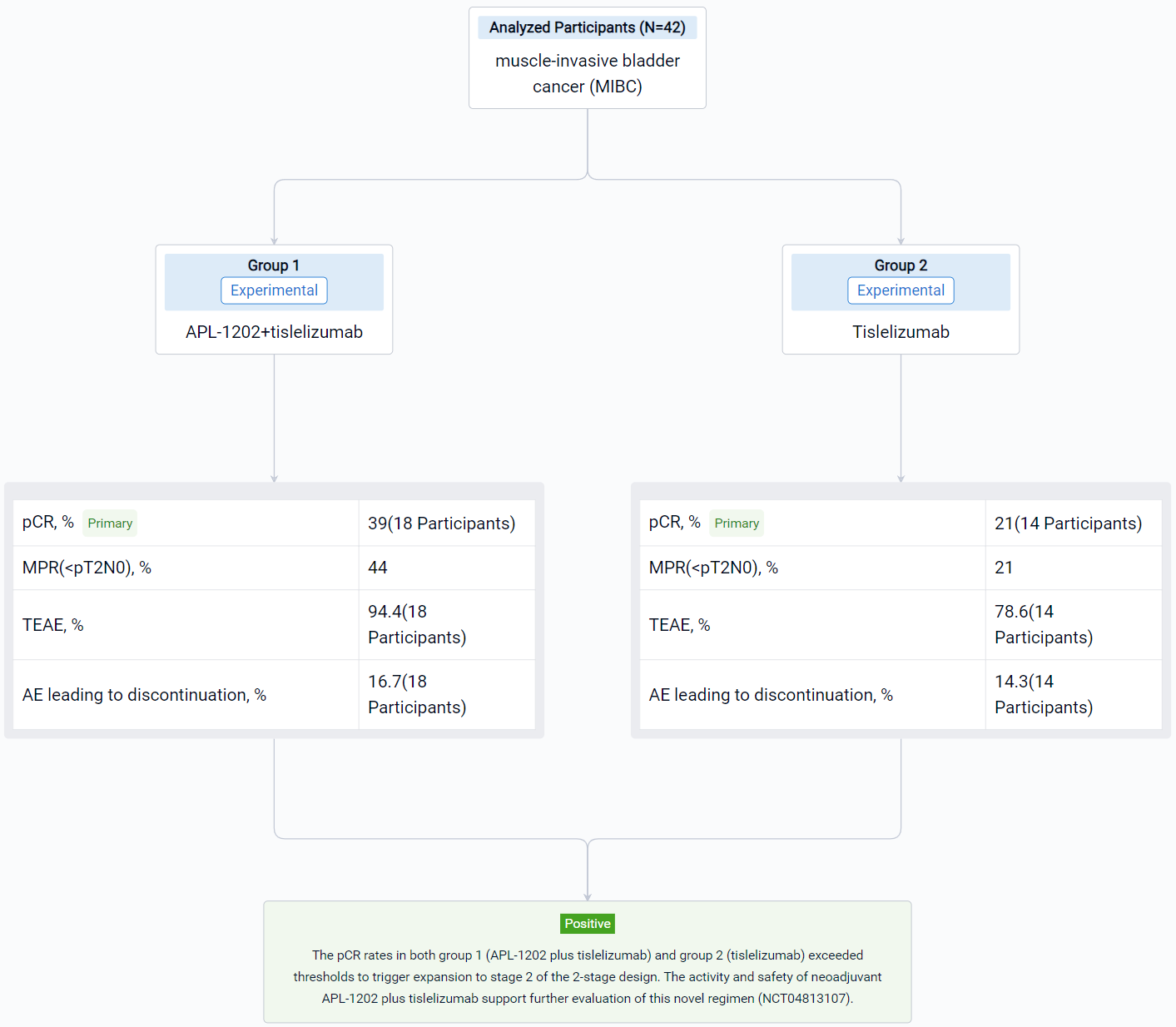
The result showed that 42 pts have enrolled and results for 32 evaluable pts for stage 1 of the 2-stage design are reported. Radical cystectomy was completed in 18/18 pts in group 1 and 13/14 pts in group 2; 1 patient who could not undergo surgery due to disease progression and 10 pts refused RC. The 32 evaluable pts consisted of 11/18 (61%) and 10/14 (72%) cT2, and 6/18 (33%) and 2/14 (14%) cT3, and 1/18 (6%) and 2/14 (14%) cT4a in group 1 and 2, respectively. PD-L1 expression was assessed using the VENTANA PD-L1 (SP263) Assay; 8/18 (44%) pts in group 1 and 7/14 (50%) in group 2 was positive. The pathological response was shown in the table. Treatment emergent adverse events (TEAEs) were reported in 17 (94.4%) pts in group 1 and 11 (78.6%) in group 2; most common (≥ 10%) TEAE of CTCAE grade ≥ 3 were anaemia (4, 22.2%), lymphocyte count decreased (3, 16.7%) in group 1 and intestinal obstruction (3, 21.4%) in group 2. AEs led to drug discontinuation in 3 (16.7%) pts in group 1 (acute kidney injury, anaemia, hepatic function abnormal) and 2 (14.3%) in group 2 (immune hyperthyroidism, COVID-19), and no treatment-related AEs led to death.
It can be concluded that the pCR rates in both group 1 (APL-1202 plus tislelizumab) and group 2 (tislelizumab) exceeded thresholds to trigger expansion to stage 2 of the 2-stage design. The activity and safety of neoadjuvant APL-1202 plus tislelizumab support further evaluation of this novel regimen (NCT04813107).
How to Easily View the Clinical Results Using Synapse Database?
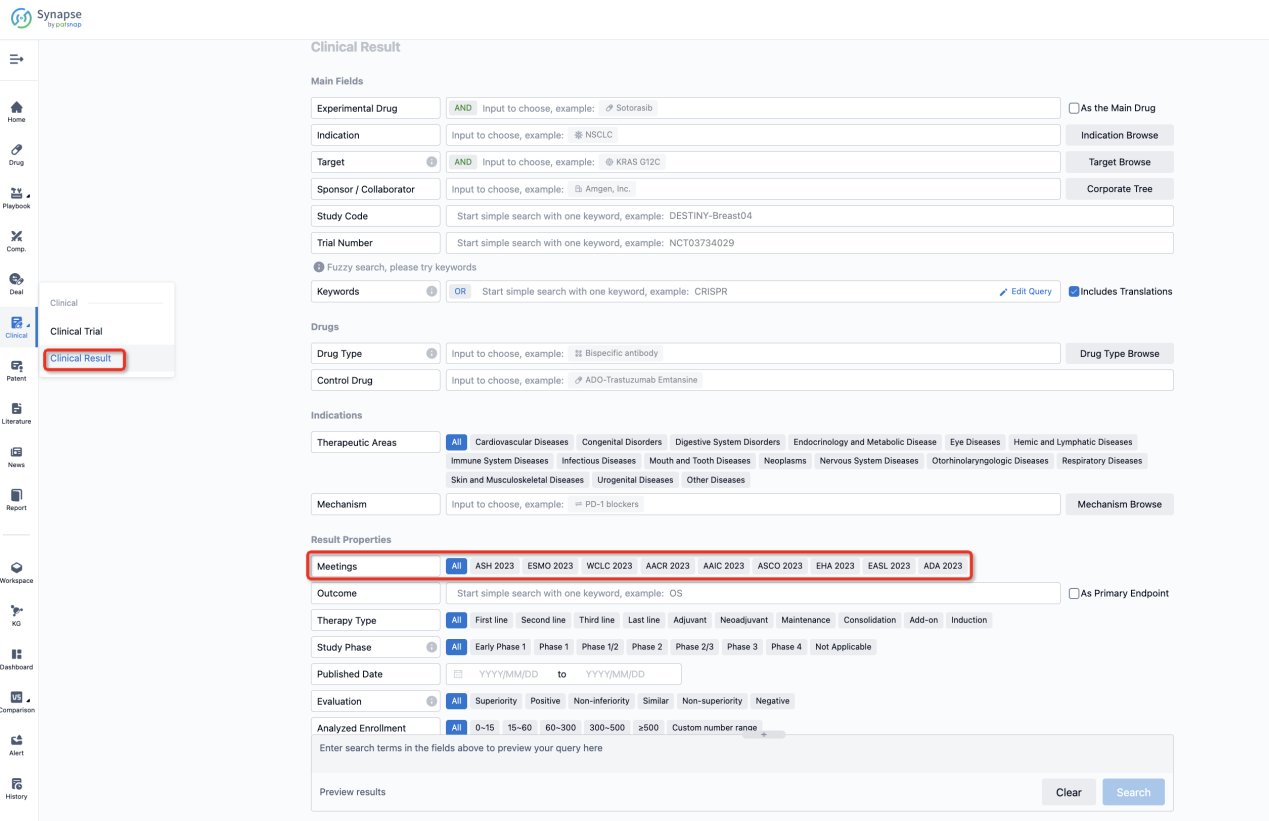
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
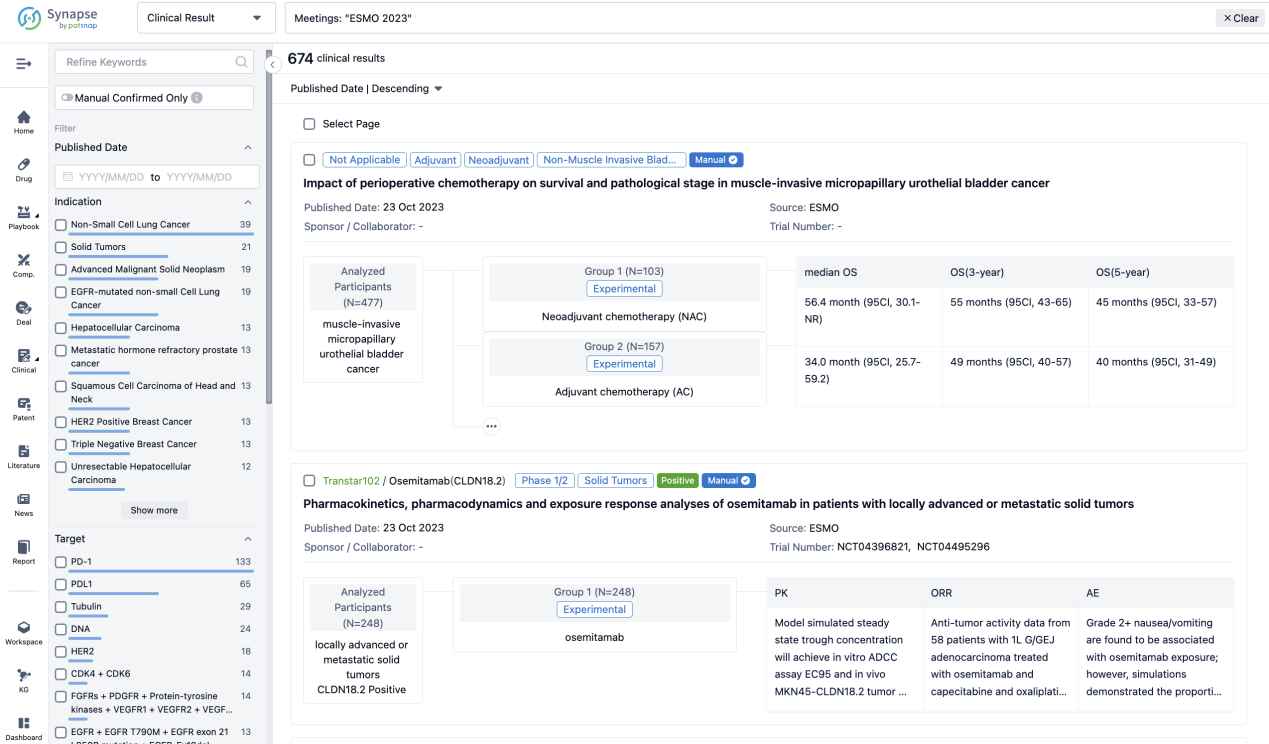
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
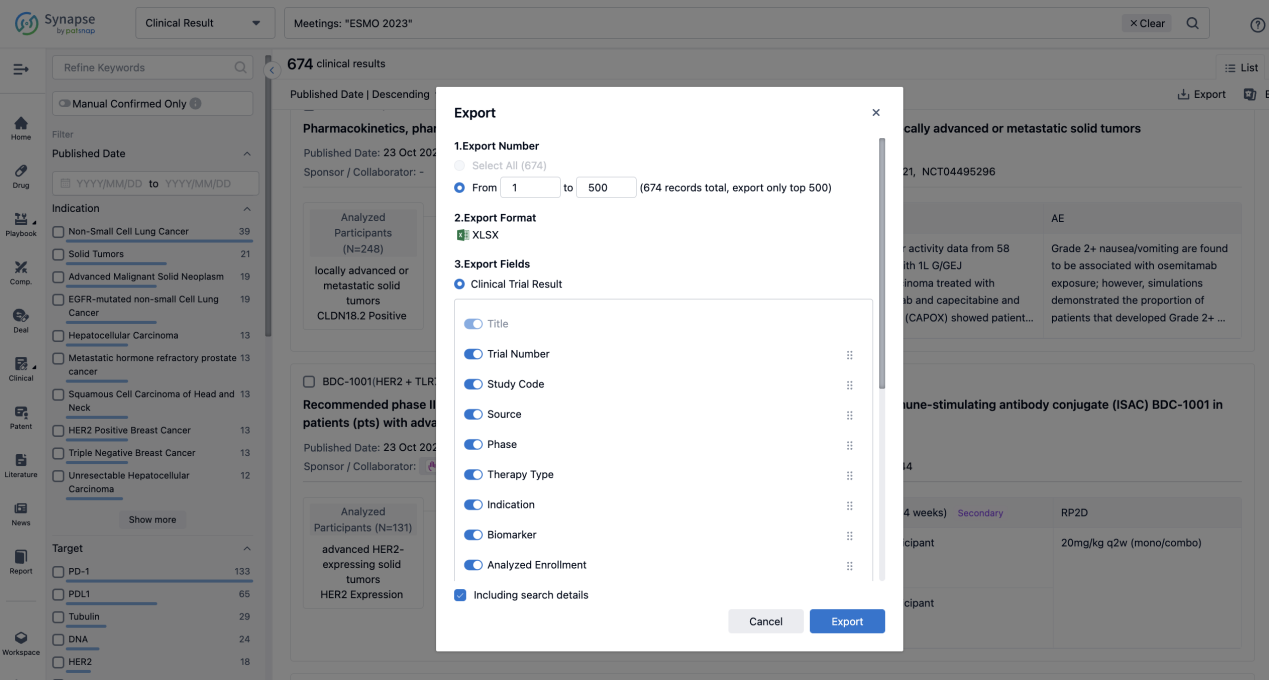
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!