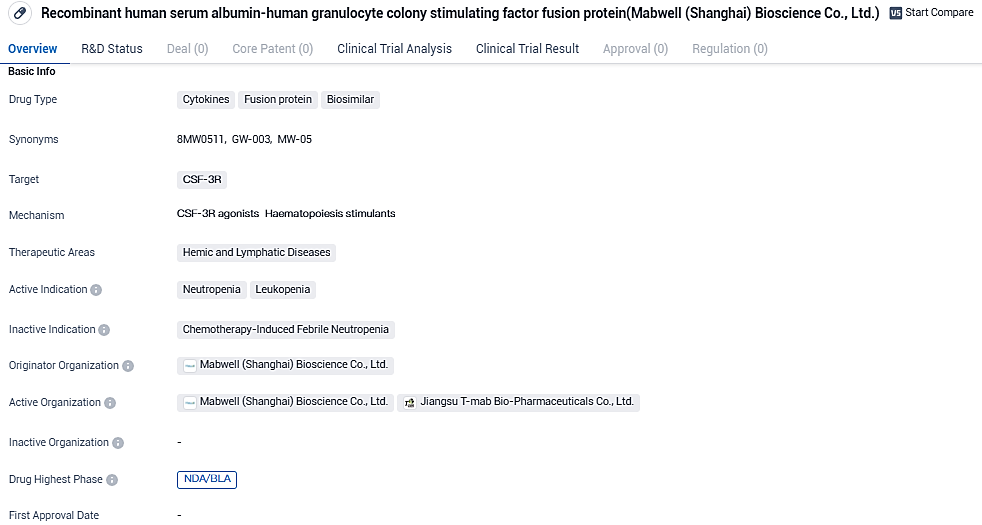
NMPA Approves Review of Mabwell’s Injectable Drug 8MW0511 for Examination
Mabwell, a pioneering company in the biopharmaceutical sector with comprehensive industry chain capabilities, has publicized the acceptance of its investigational new drug filing by the National Medical Products Administration. The drug, a novel recombinant fusion protein that combines human serum albumin with human granulocyte colony-stimulating factor, is designed for injectable use and is identified by its research and development code: 8MW0511.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The therapeutic agent 8MW0511 has been formulated for adult patients facing non-myeloid cancerous growths, aiming to diminish the potential for infection occurrences characterized by fever and a significant reduction in neutrophil count. This condition can arise following the administration of certain chemotherapeutic agents known to suppress bone marrow activity and predispose patients to febrile neutropenia.
Structured as a primary category biological therapeutic compound, 8MW0511 is a novel, extended-duration form of G-CSF created by Mabwell, a company holding proprietary rights to its formulation. The production methodology for 8MW0511 involves a pioneering combination of a G-CSF variable non-homologous with an endogenous protein - human serum albumin - using advanced genetic bonding mechanisms. This innovative approach notably hinders the normal route of receptor-driven elimination specific to G-CSF, thereby extending its biological half-life, cutting down the necessity for frequent dosages, and enhancing patient adherence during treatments.
Utilizing a sophisticated yeast-based bioprocessing system, the production of 8MW0511 achieves a higher consistency in its output, streamlines the manufacturing workflow, and is anticipated to bring down overall expenditure by circumventing the need for polyethylene glycol (PEG) based modifications.
Clinical findings from a Phase III trial, unveiled at the European Society for Medical Oncology conference in 2023, affirm the efficacy of 8MW0511 as being on par with the benchmark treatment with PEG-rhG-CSF. Considerable improvements in the rate and severity of Grade 4 neutropenia were observed. The treatment's safety spectrum was reported to be agreeable and akin to that of the comparator group, denoting a well-regulated safety aspect and favorable patient tolerance levels.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
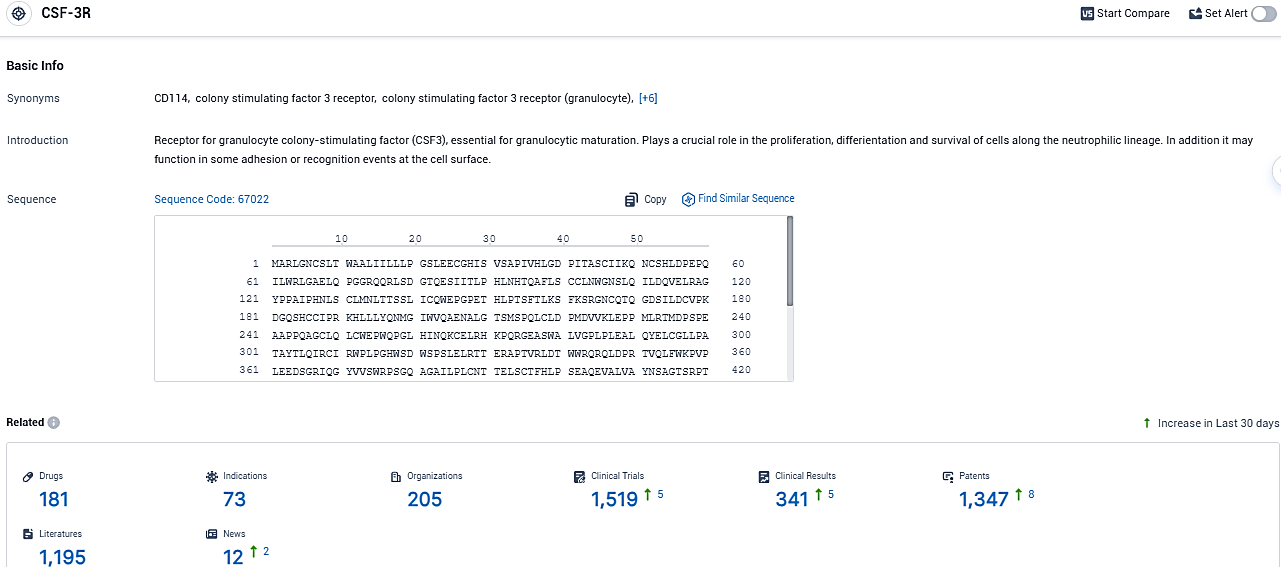
According to the data provided by the Synapse Database, As of December 28, 2023, there are 181 investigational drugs for the CSF-3R target, including 73 indications, 205 R&D institutions involved, with related clinical trials reaching 1519, and as many as 1347 patents.
8MW0511 is a cytokine fusion protein and biosimilar that targets the CSF-3R receptor. It is primarily used for the treatment of hemic and lymphatic diseases, specifically neutropenia and leukopenia. The drug is currently in the highest phase of development, NDA/BLA, both globally and in China, indicating its advanced stage of development.