Exploring the Latest Potential "First-in-Class" Therapy Deal by Karuna Therapeutics: A Guide to Rapidly Accessing Transaction Insights
Recently, Bristol Myers Squibb (BMS) and Karuna Therapeutics announced that they have reached a definitive acquisition agreement. According to the agreement, Bristol Myers Squibb agrees to acquire Karuna for a total value of approximately 14 billion U.S. dollars. Through this acquisition, Bristol Myers Squibb will gain Karuna's potential "first-in-class" therapy, KarXT (xanomeline-trospium), which is used for the treatment of schizophrenia in adults. The New Drug Application (NDA) for this medication has been accepted by the U.S. FDA, with a PDUFA target date of September 26, 2024.
About KarXT
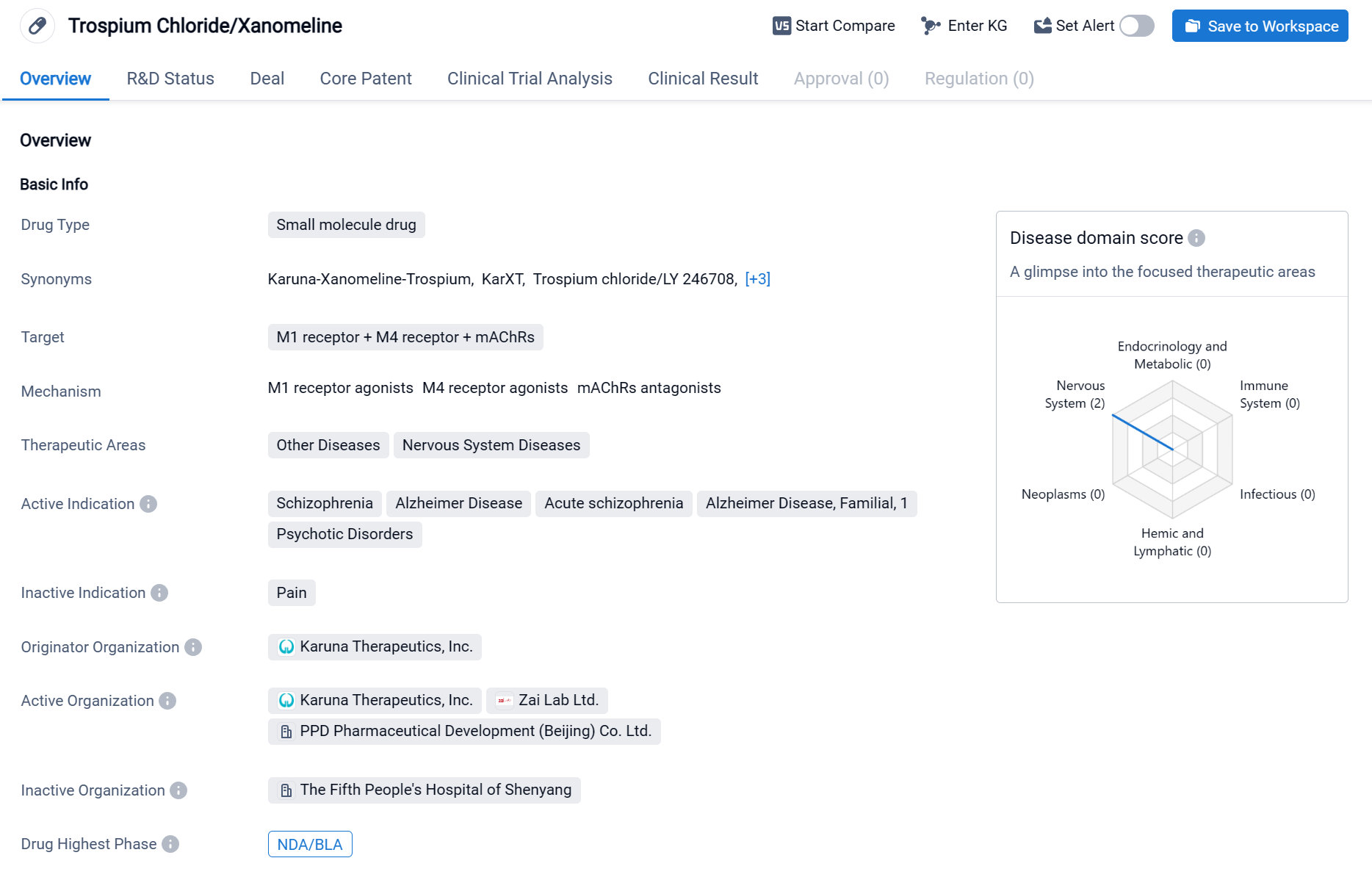
KarXT is a small molecule drug that targets the M1 receptor, M4 receptor, and mAChRs in the field of biomedicine. KarXT is composed of two active ingredients, xanomeline and trospium chloride, which aim to activate muscarinic acetylcholine receptors in the brain while reducing the effect on peripheral muscarinic acetylcholine receptors. It is primarily used for the treatment of various diseases related to the nervous system, including schizophrenia, Alzheimer's disease, acute schizophrenia, Alzheimer's disease familial, and psychotic disorders. Click the image below to directly embark on the exploration journey with the KarXT!
Currently, KarXT has reached the highest phase of development, known as NDA/BLA, on a global scale. The result from phase Ⅲ clinical trial NCT04659161 showed that KarXT demonstrated statistically significant and clinically meaningful improvements in positive and negative symptoms of schizophrenia compared to placebo, as measured by primary and secondary endpoints. KarXT was generally well tolerated and not associated with many adverse events typically associated with current antipsychotics, including somnolence, weight gain and extrapyramidal symptoms.
About Karuna Therapeutics
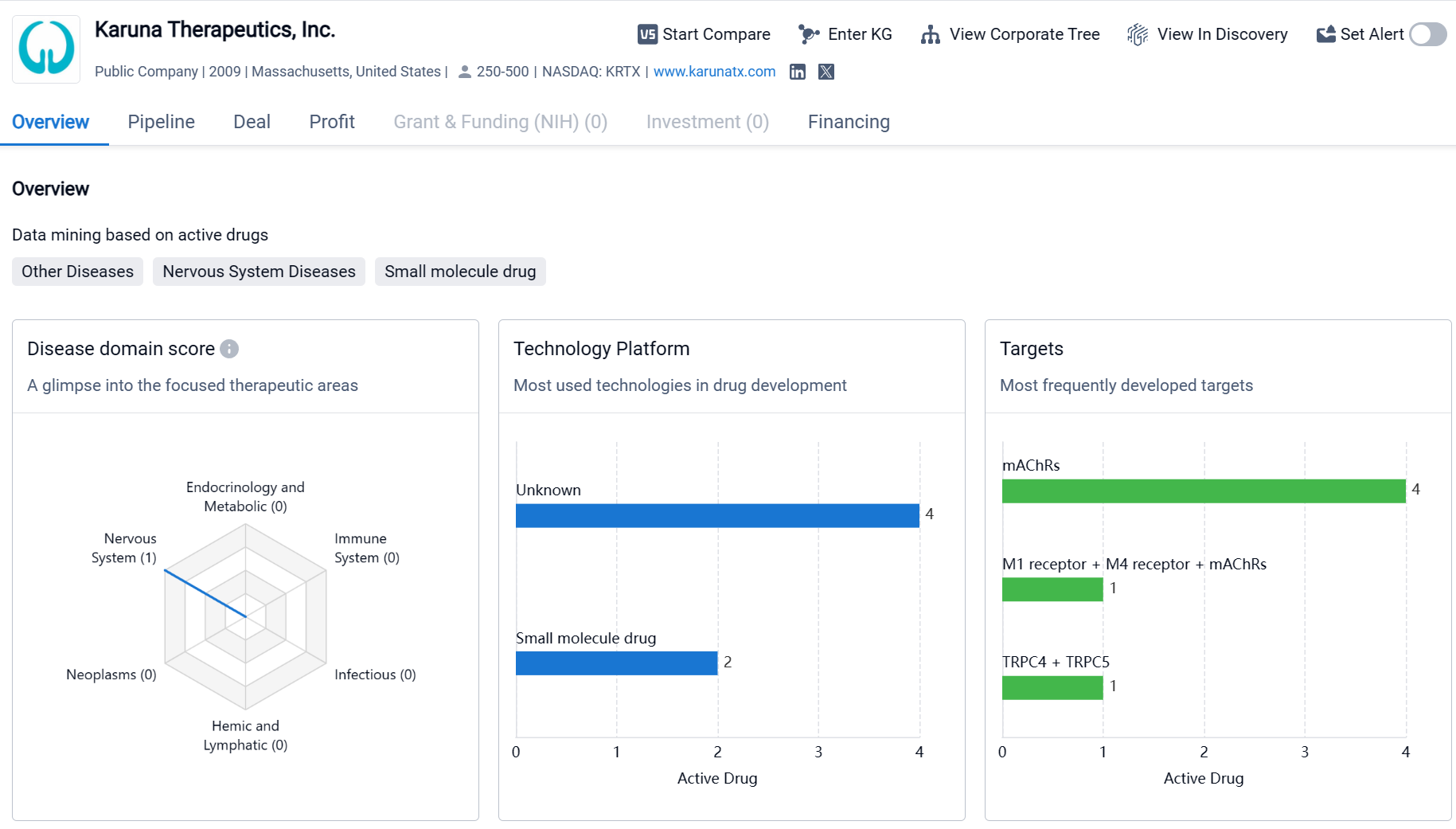
Karuna Therapeutics, Inc. is a biopharmaceutical company that focuses on the development of therapeutics for various diseases, with a particular emphasis on nervous system diseases. The company has a diverse portfolio of drugs targeting both other diseases and nervous system diseases. They have a strong focus on developing drugs that target muscarinic acetylcholine receptors (mAChRs), with four drugs in this category. Additionally, Karuna Therapeutics is exploring the potential synergistic effects of targeting multiple receptors simultaneously and is also investigating targets beyond traditional receptors. While the company has several drugs in the pipeline, they are still in the early stages of development, with most of them in the preclinical stage. Karuna Therapeutics has one drug in Phase 1 and one drug in the NDA/BLA stage, indicating progress towards potential commercialization. However, it is important to note that as of now, none of their drugs have received approval for marketing.
How to get the latest progress on drug deals?
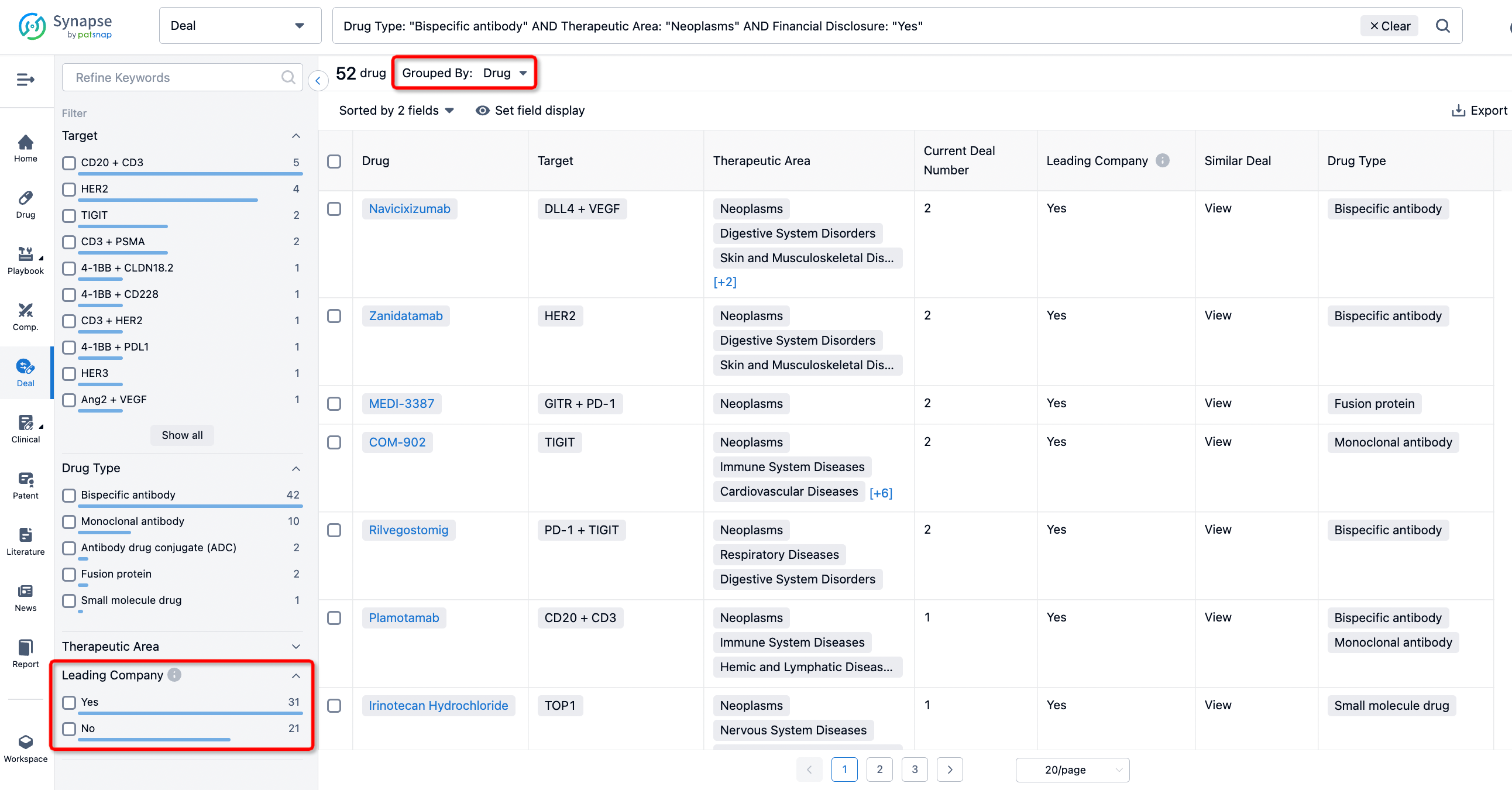
If you would like to access the latest transaction event information, you can click on the 'Deal' module from the homepage of the Synapse database. Within the Deal module, you can search for global pharmaceutical transaction information using labels such as Drugs, Organization, Target, Drug Type, Deal Date.
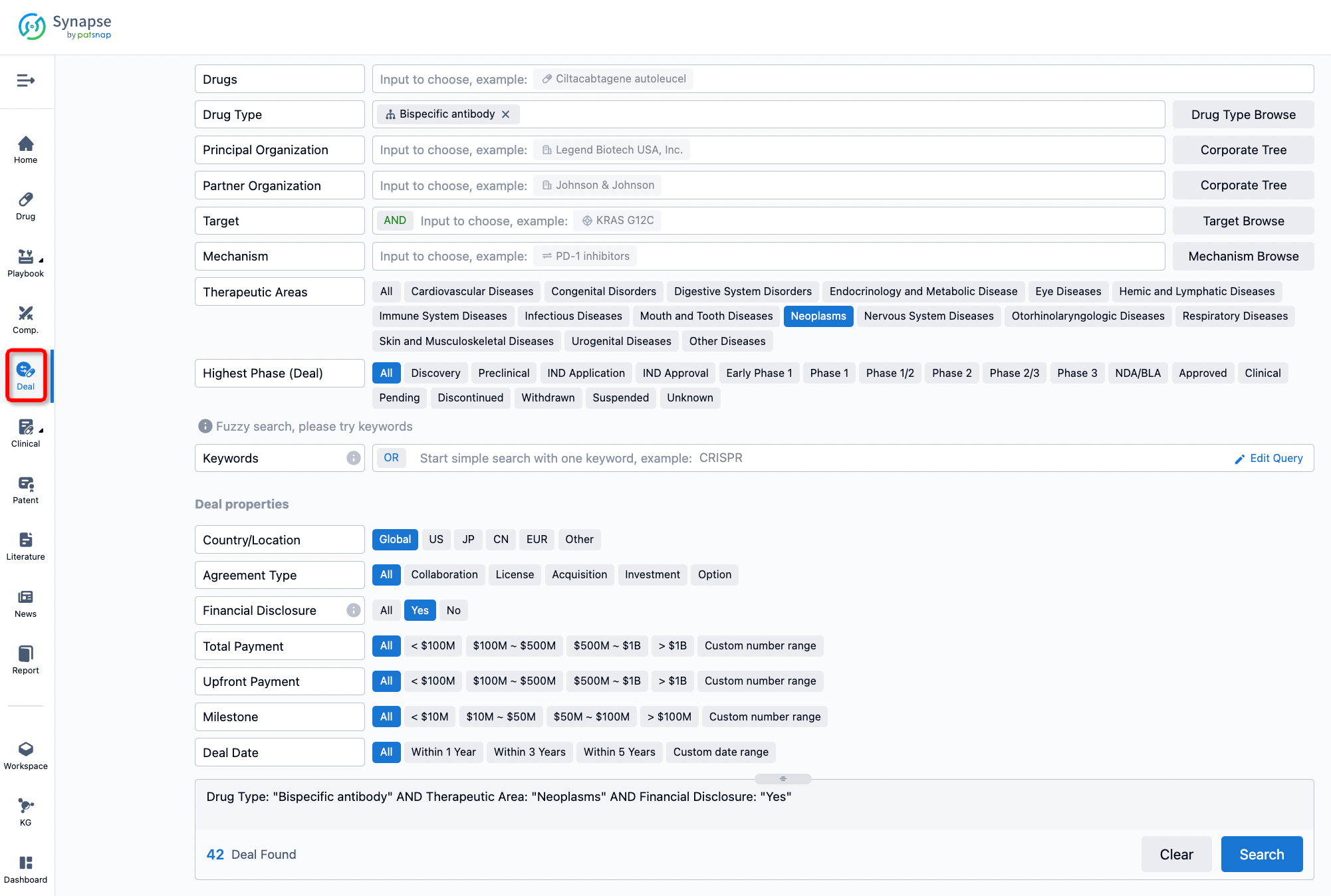
Furthermore, you can obtain the original link to the transaction coverage by clicking on the "Deal Name."
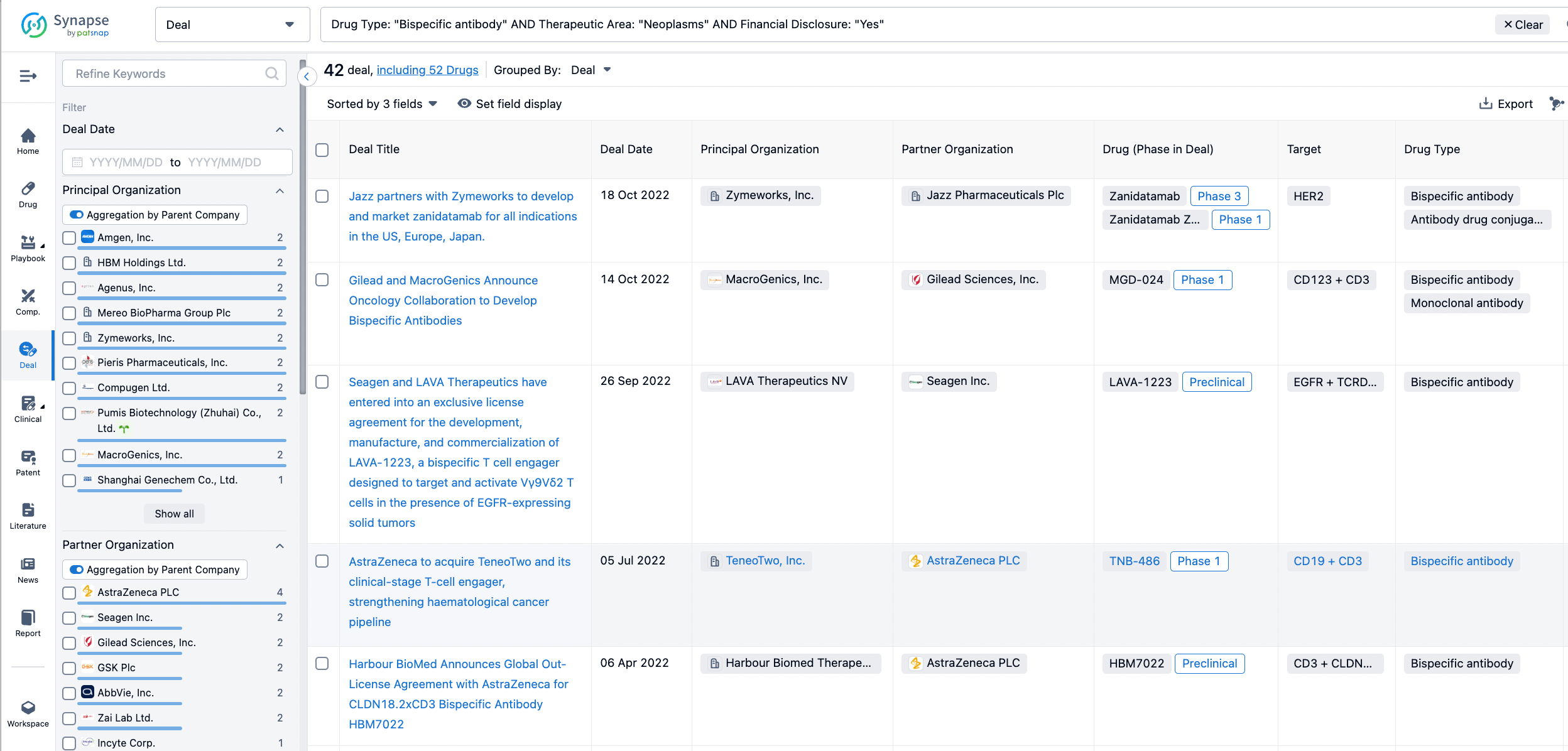
In the analysis view, you can see the most active assignors, assignees, popular targets, and other dimensions of analysis, as well as the distribution of research and development statuses at the time of the transaction, to help you better understand the search results.
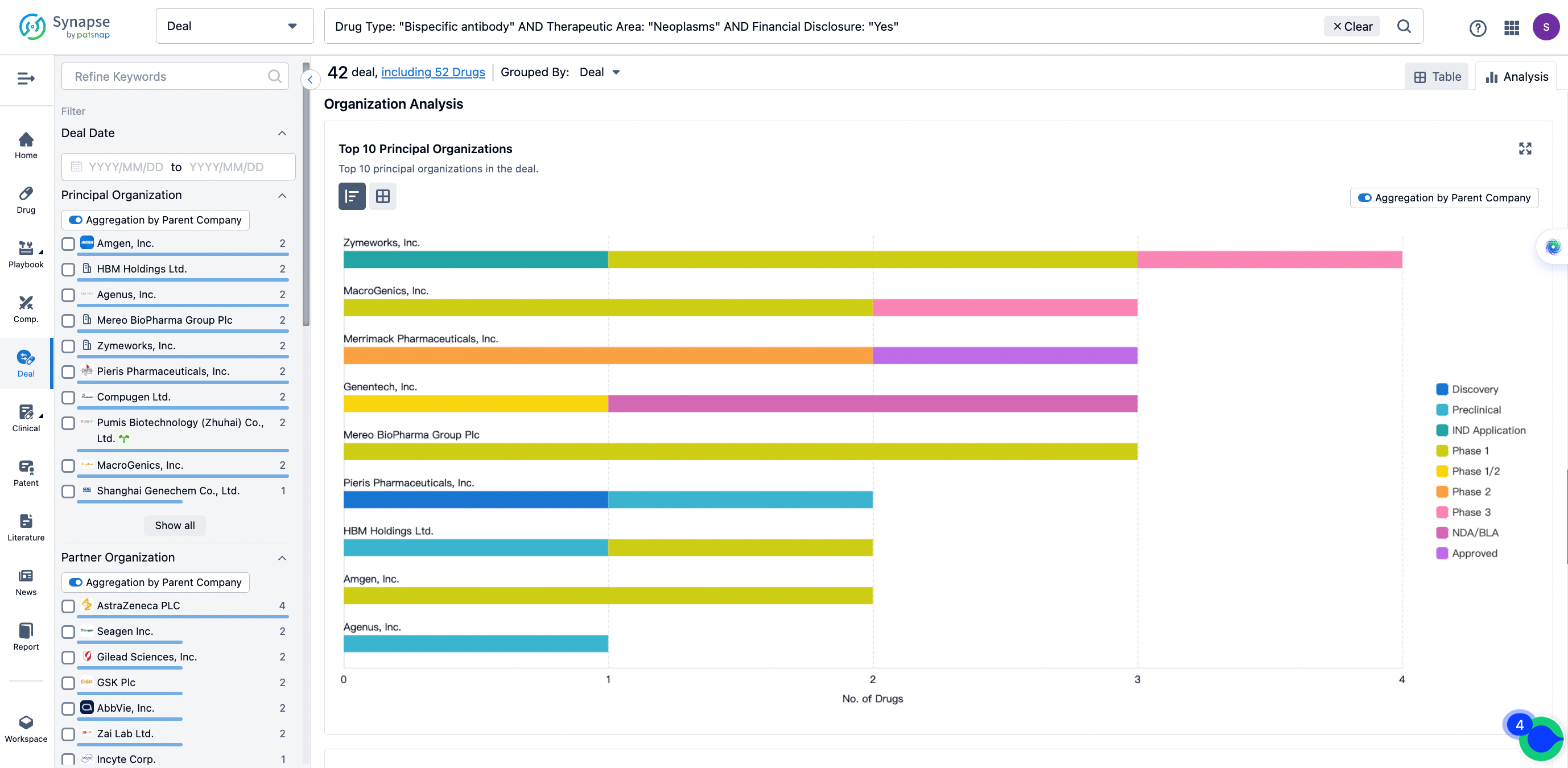
The Synapse database also supports the ability to view current transactions from the dimension of "drugs" (by selecting "drugs" from the "Adjust Dimension" dropdown menu above). Targeting transactions involving renowned pharmaceutical companies that are of interest to the industry, such as Merck, Roche, etc., Synapse has identified a group of "leading companies" through drugs that have achieved global sales exceeding 1 billion US dollars in 2022. Transactions involving drugs from these leading companies can be filtered by clicking on the "Leading Company" tag on the left-hand side.
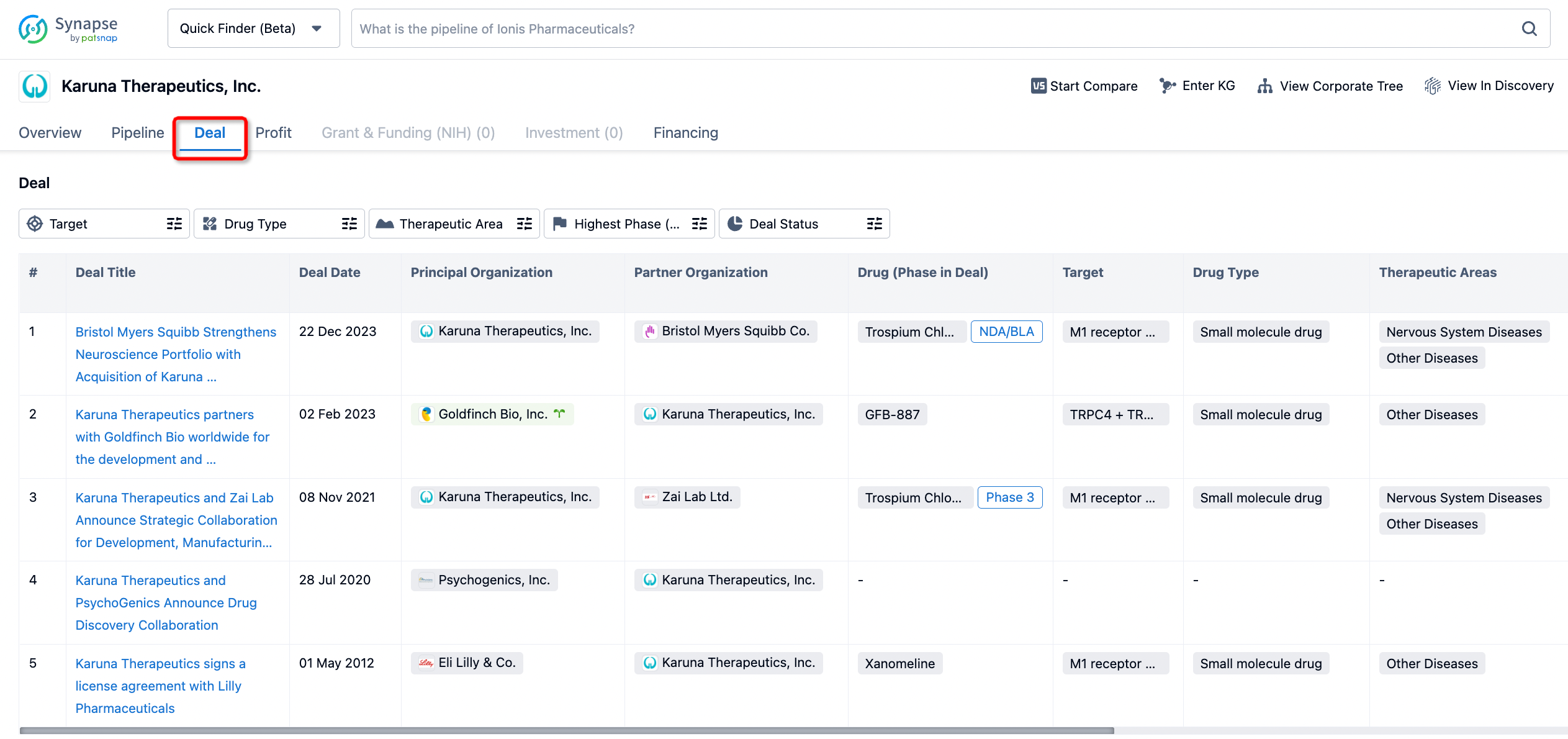
In addition to the drug transaction module, you can also view related transaction history on the drug detail page and the institution detail page.

Click on the image below to embark on a brand new journey of drug discovery!