Novo Holdings Leads $100M Series C Funding for Asceneiron's Alzheimer's Innovation
Novo Holdings, a prominent investor in the life sciences sector, has disclosed that it spearheaded a $100 million Series C funding round for Asceneuron. Asceneuron is a biotech company in the clinical stage, focused on creating small molecules aimed at tau protein aggregation, which is a factor in neurodegenerative diseases. The funds will be allocated to progress Asceneuron’s leading asset, ASN51, into Phase 2 clinical trials for Alzheimer's disease treatment.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
ASN51 is an orally administered small molecule drug developed to inhibit OGA, an enzyme involved in tau protein aggregation. By blocking tau protein aggregation, ASN51 aims to decelerate the progression of Alzheimer's disease. Inhibition of OGA has also demonstrated promising potential to prevent the aggregation of proteins central to other neurodegenerative diseases such as Parkinson's disease and amyotrophic lateral sclerosis.
The distinct mode of action and convenient oral formulation of ASN51 make it an ideal treatment for Alzheimer's disease patients. Asceneuron has completed five Phase 1 clinical trials, showing complete central nervous system uptake and high OGA enzyme occupancy, suggesting its potential to stand out from competitors. Asceneuron plans to initiate its first Phase 2 clinical trial later this year.
Naveed Siddiqi MD, Senior Partner, Venture Investments, Novo Holdings said: “Alzheimer’s disease is experiencing a pivotal moment. Millions suffer from this devastating illness, and therapeutic options are very limited. Validated biomarkers are enabling more focused and rapid development. We are now seeing the approval of the first disease-modifying antibody-based injectable therapies. Asceneuron’s groundbreaking oral small molecule drug targeting intracellular tau offers the potential for a paradigm shift in how this neurodegenerative disease is treated.”
Barbara Angehrn Pavik, Chief Executive Officer of Asceneuron, remarked: “This high-quality life science investor syndicate led by Novo Holdings further corroborates the potential of our OGA inhibitor pipeline and our leadership in the field of tauopathies. We are thrilled to advance our leading asset ASN51 into Phase 2 clinical development, recognizing its potential to significantly expand treatment options for Alzheimer’s disease patients.”
The financing round was led by Novo Holdings, with additional new investment from EQT Life Sciences Dementia Fund, OrbiMed, and SR One, alongside participation from existing investors M Ventures and Sofinnova Partners.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
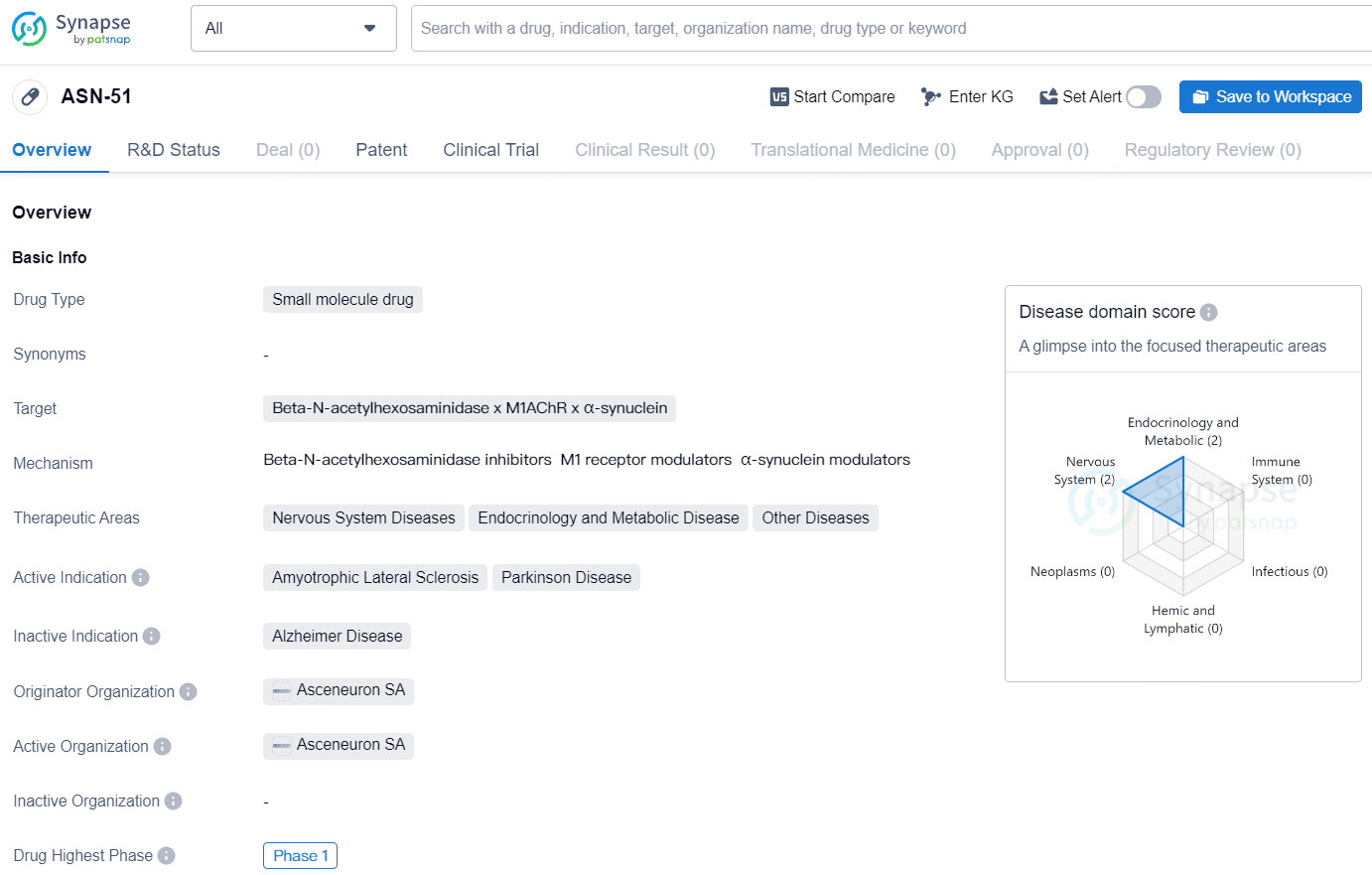
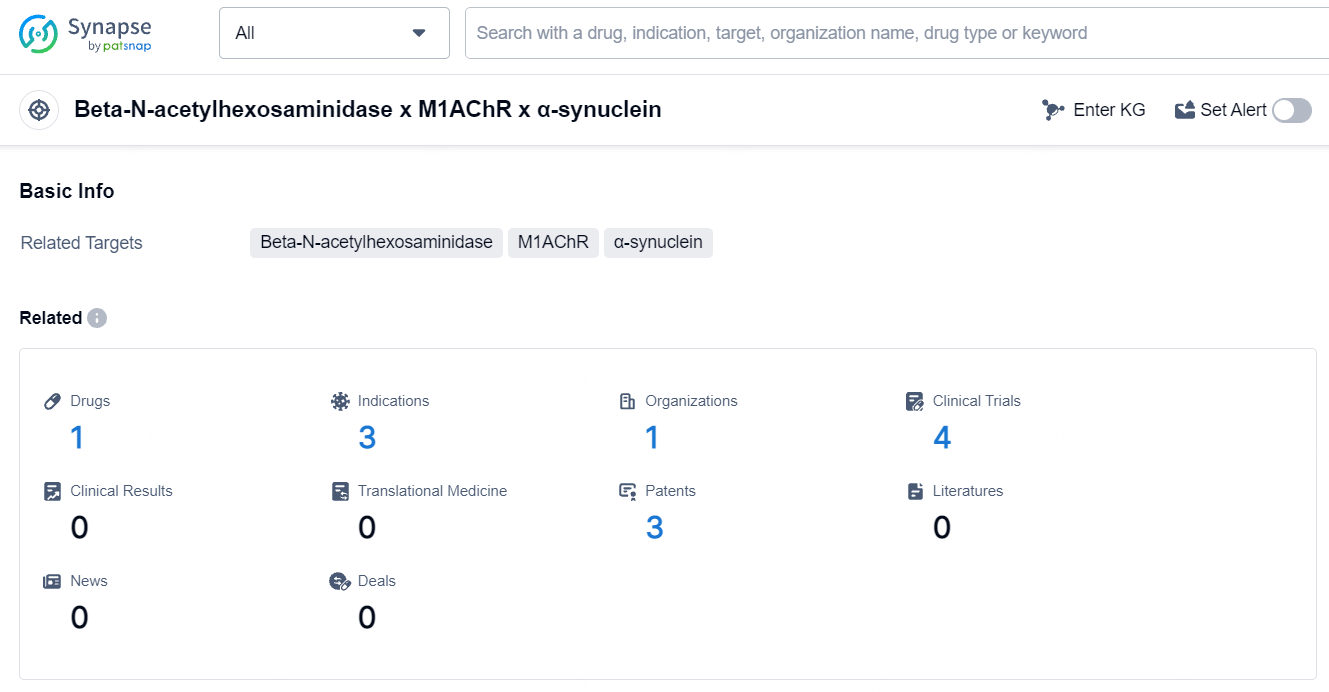
According to the data provided by the Synapse Database, As of July 22, 2024, there are 1 investigational drug for the Beta-N-acetylhexosaminidase and M1AChR and α-synuclein target, including 3 indications, 1 R&D institution involved, with related clinical trials reaching 4, and as many as 3 patents.
ASN-51 is a small molecule drug designed to target Beta-N-acetylhexosaminidase, M1AChR, and α-synuclein. The drug is intended to treat a range of diseases within the Nervous System, Endocrinology and Metabolic Disease, and other areas. The active indications for ASN-51 include Amyotrophic Lateral Sclerosis and Parkinson Disease. At the time of reporting, the drug is in Phase 1 of clinical trials, indicating that it has progressed beyond basic safety testing and is now being tested for effectiveness and potential side effects in humans.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!