Evommune Reports Successful Early Clinical Trial Outcomes for MRGPRX2 Blocker (EVO756)
Evommune, Inc., a biotechnology company at the clinical stage focusing on discovering and developing innovative treatments for immune-mediated inflammatory conditions, reported positive findings from its initial proof-of-concept study in humans with EVO756.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
By inhibiting MRGPRX2 activation and mast cell degranulation, EVO756 may offer a groundbreaking oral treatment option for numerous conditions driven by mast cells. Evommune plans to showcase extensive trial data at a peer-reviewed scientific conference in autumn 2024.
"The results from our proof-of-concept trial surpassed our expectations, prompting us to plan several clinical trials for EVO756," stated Eugene Bauer, M.D., Chief Medical Officer at Evommune. "We intend to launch a Phase 2b study in chronic spontaneous urticaria patients within the first half of 2025. We believe we have a novel, potent, and selective oral agent that could be administered once daily, presenting extensive opportunities for patients experiencing various mast cell-mediated conditions."
The pharmacodynamic impact of EVO756 on mast cell degranulation was examined through a skin challenge test. In this test, icatibant, a well-known MRGPRX2 receptor ligand, was injected intradermally, producing noticeable skin reactions in all participants in the multiple ascending dose study. Several experimental approaches have established that mast cell degranulation triggered by icatibant mirrors the changes tied to MRGPRX2 disease-relevant endogenous ligands. This segment of the study enabled a controlled evaluation of target engagement and activity, akin to the potential effects of EVO756 versus a placebo in inducible urticarias.
"EVO756’s compelling safety profile and potential for once-daily oral dosing sustain the excitement surrounding this innovative class of therapies for inflammatory diseases. Consistent with our expectations, the data further back the hypothesis that blocking the MRGPRX2 receptor and its downstream effects could address the root cause of inflammation, delivering superior relief compared to current treatments," commented Sarbjit Saini, M.D., Professor of Medicine at Johns Hopkins University in Baltimore, Maryland.
EVO756 is a powerful, highly selective small molecule antagonist of mas-related G-protein coupled receptor X2 (MRGPRX2). MRGPRX2 is predominantly located on mast cells and peripheral sensory neurons and can induce IgE-independent activation through multiple ligands, which may result in various symptoms depending on the affected tissue.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
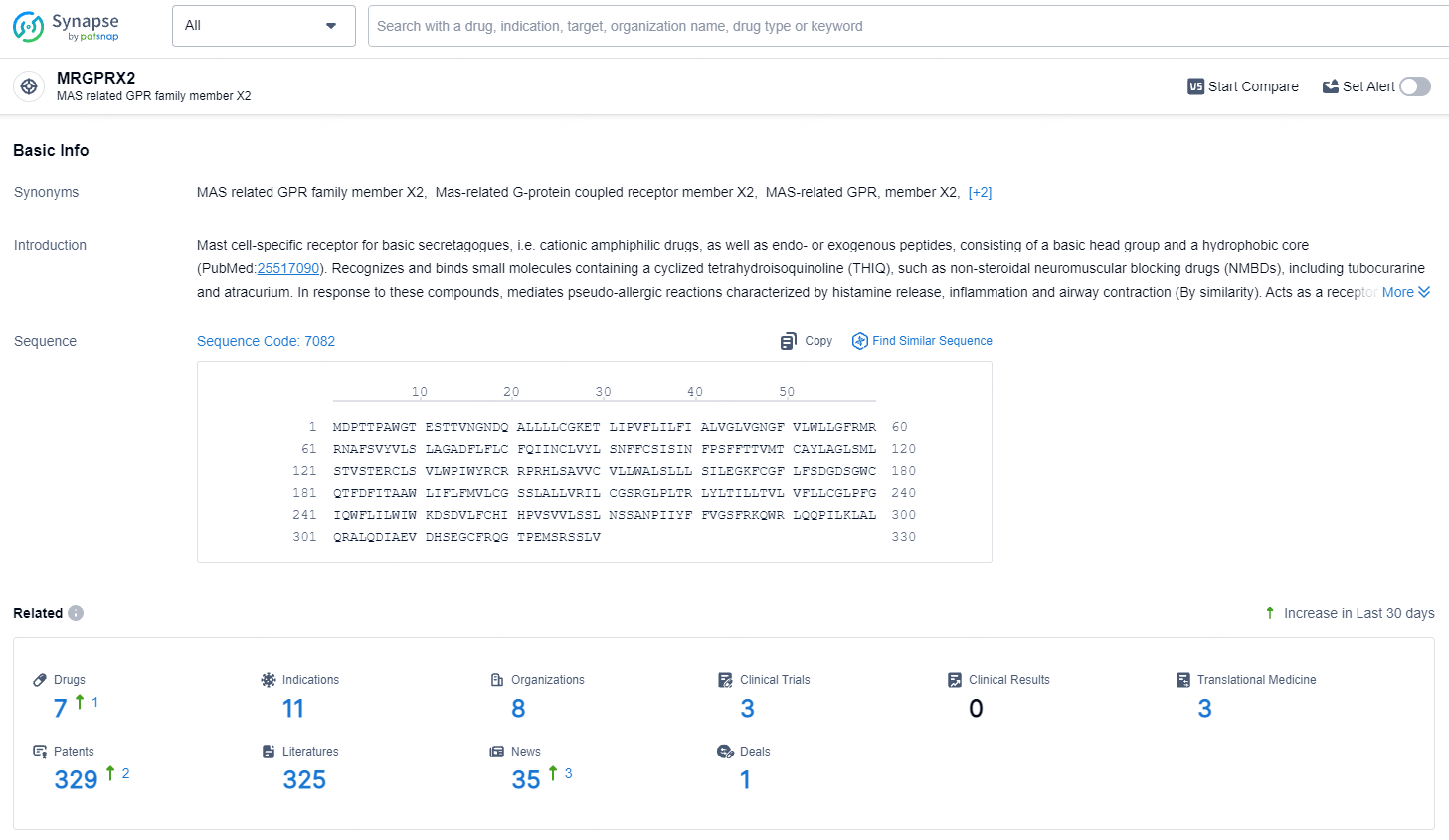
According to the data provided by the Synapse Database, As of July 22, 2024, there are 7 investigational drugs for the MRGPRX2 target, including 11 indications, 8 R&D institutions involved, with related clinical trials reaching 3, and as many as 329 patents.
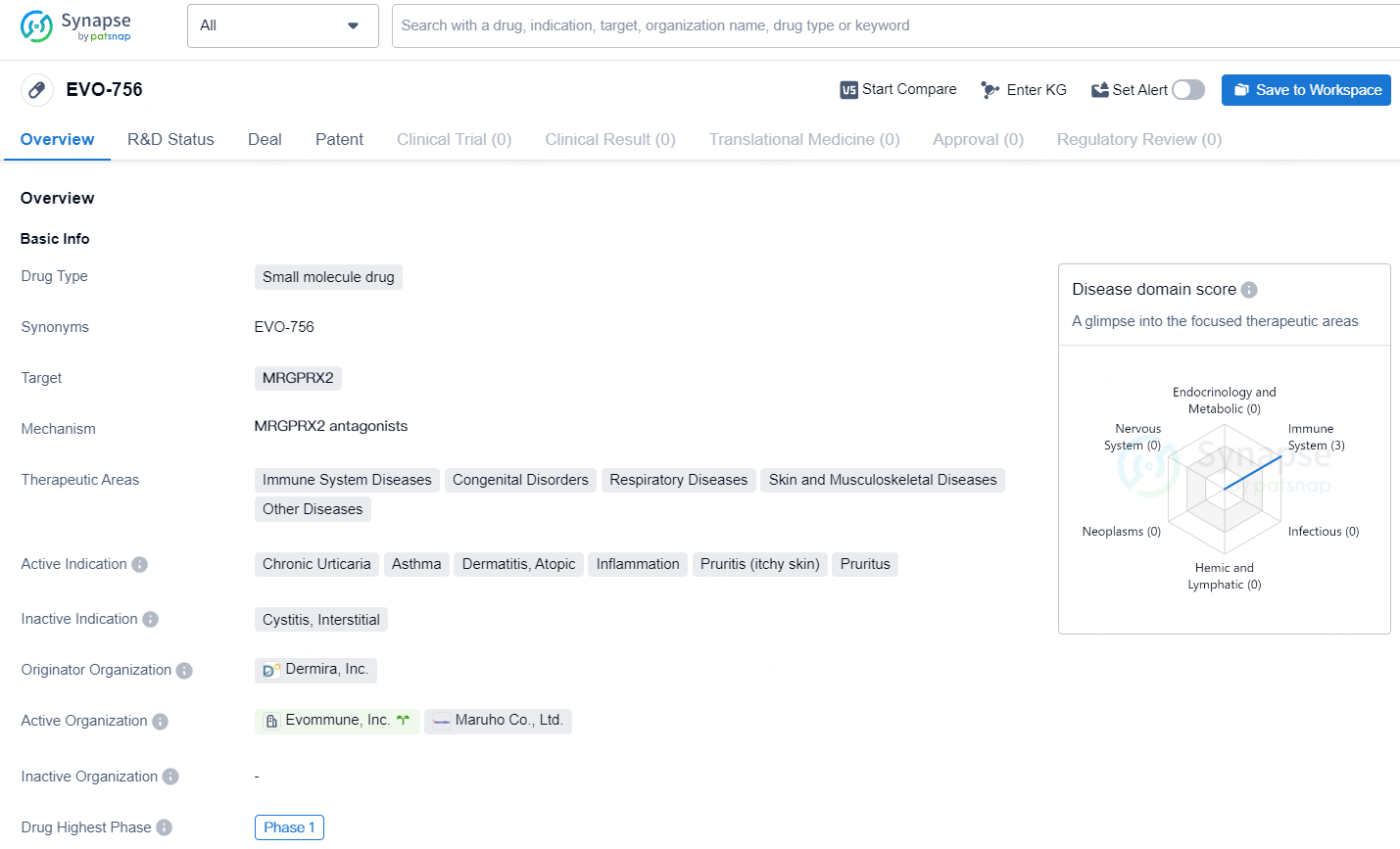
EVO-756 is a small molecule drug targeting MRGPRX2 and showing promise in addressing a wide range of therapeutic areas and active indications. EVO756 has the potential to be a first-in-class oral treatment for a variety of mast cell mediated diseases. In addition, due to its unique function on peripheral sensory neurons, EVO756 could provide fast relief of itch associated with inflammatory diseases, such as atopic dermatitis.