Novo Nordisk's Mim8 Treatments Significantly Reduce Bleeding Episodes in Haemophilia A Patients in Frontier 2 Study
Novo Nordisk recently disclosed the main outcomes of the FRONTIER 2 study. This pivotal phase 3a trial, lasting 26 weeks, was an open-label, randomized, controlled, multi-arm study involving 254 participants. The study assessed the effectiveness and safety of Mim8 administered subcutaneously either once a week or once a month, compared to no prophylaxis and also against previous prophylactic treatments with coagulation factor in individuals aged 12 and above who have haemophilia A, with or without inhibitors.
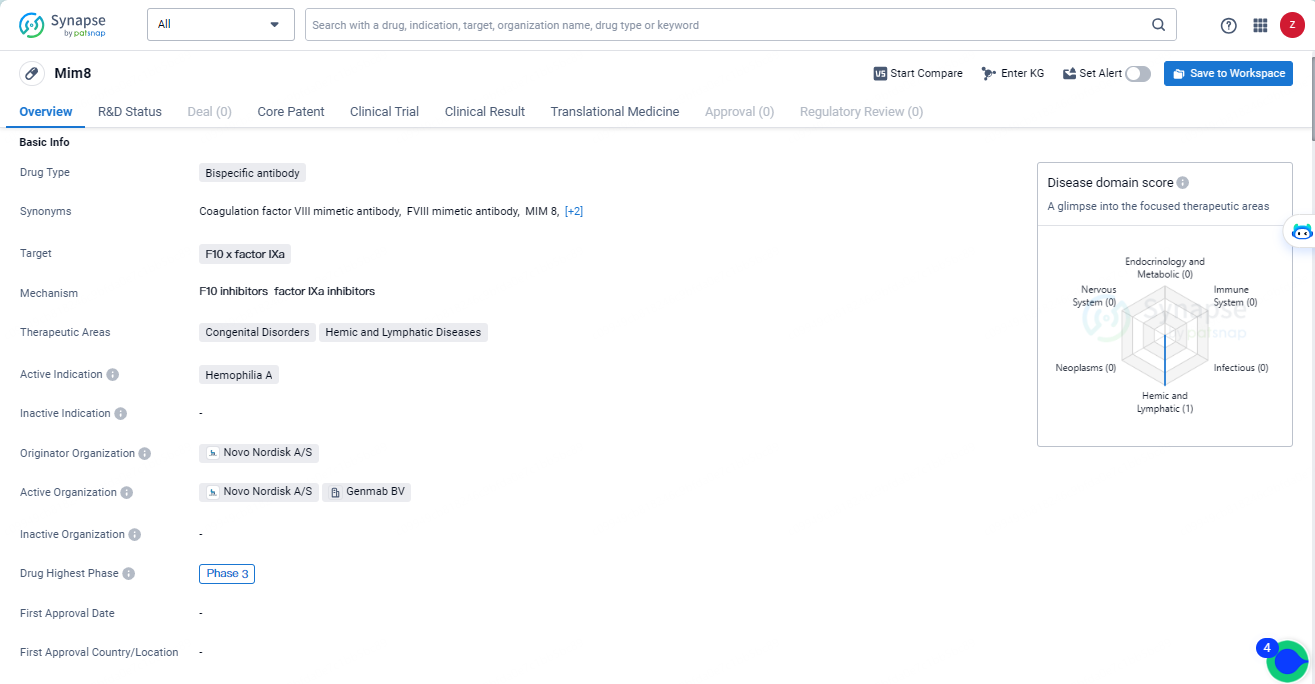
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The study successfully met its primary goals, showing a statistically significant and superior decrease in the number of bleeding episodes treated when using either once-weekly or once-monthly Mim8 compared with either no prophylaxis or previous coagulation factor prophylaxis.
For individuals who had not received any prior prophylaxis, administration of Mim8 either once a week or once a month led to reductions in treated bleeding episodes by 97% and 99%, respectively, when measured against those without any prophylaxis. Moreover, 86% of subjects receiving weekly Mim8 doses and 95% of those on a monthly schedule experienced no bleeding episodes, in stark contrast to those who had no prophylaxis, all of whom experienced bleeds.
Within the group with a history of coagulation factor prophylaxis, the once-weekly and once-monthly dosage of Mim8 resulted in 48% and 43% decreases in bleeding, respectively, when compared to their previous treatments. Furthermore, 66% of participants on the weekly regimen and 65% on the monthly regimen reported no bleeding occurrences.
Martin Holst Lange, executive vice president for Development at Novo Nordisk, expressed satisfaction with these outcomes from the FRONTIER 2 clinical trial, noting that the data confirm Mim8's efficacy and safety in preventing bleeds in individuals with haemophilia A across different dosing schedules.
Martin Holst Lange also mentioned, “The availability of convenient dosing schedules either weekly or monthly meets the varied needs of those managing haemophilia A, offering them greater flexibility and choice, whether they have inhibitors or not.”
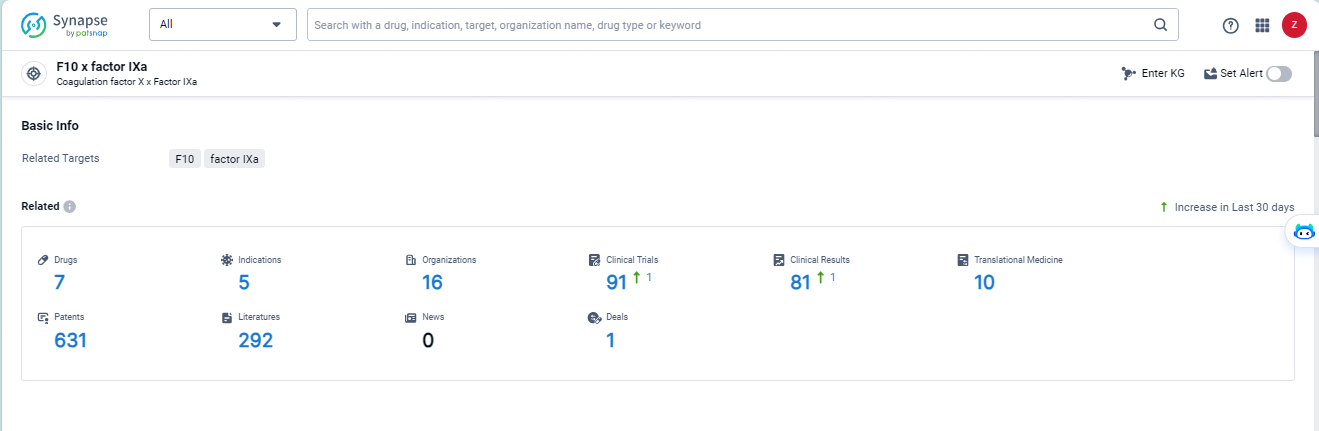
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 14, 2024, there are 7 investigational drugs for the F10 x factor IXa targets, including 5 indications, 16 R&D institutions involved, with related clinical trials reaching 91, and as many as 631 patents.
Mim8 is currently in Phase 3 trials globally and in China, indicating its advanced stage of development. If approved, Mim8 has the potential to offer a novel therapeutic approach for patients with Hemophilia A, addressing the deficiency of clotting factor VIII. Mim8 has already undergone rigorous testing in earlier phases and has shown promising results, leading to its advancement to the final stage of clinical trials.