Nuclear Medicine: New Trends in Antitumor Drugs
Radioligand therapy (RLT), as a part of radiopharmaceuticals, delivers high-energy radiation directly to cancer cells by targeting their structural or biochemical mutations, while minimizing damage to surrounding tissues. Theoretically, this could reduce side effects and improve efficiency. These therapies also possess imaging characteristics related to the same cellular structure mutations, allowing physicians to image patients before starting treatment and assess the drug's targeting efficacy—an advantage not present in current treatment methods.
·The market for radioligand therapy (excluding imaging agents) is projected to grow from approximately $750 million in total sales in 2022 to about $5.5 billion by 2028, with a compound annual growth rate of around 40%.
· Currently, there are 82 radioligand therapy assets in clinical development, covering at least 10 types of solid tumors and hematological malignancies, involving 15 different isotopes. Combination therapies represent an active area of research.
· Due to their higher efficacy and reduced off-target effects, alpha-emitting radioisotopes have attracted increasing interest in research and development.
· Developing and commercializing radioligand therapies differ from traditional pharmaceutical products, presenting a set of unique challenges that must be comprehensively understood and addressed to successfully bring radioligand therapies to market.
· For manufacturers aiming to play a leading role in this market, understanding the necessary capabilities, the optimal timing for investment, and the scale of investment is also crucial.
Radioligand therapy (RLT) is an emerging targeted therapeutic approach under the trend of precision medicine. It consists of three critical components: a ligand targeting cancer cells, a radioactive isotope, and a linker that connects the two.
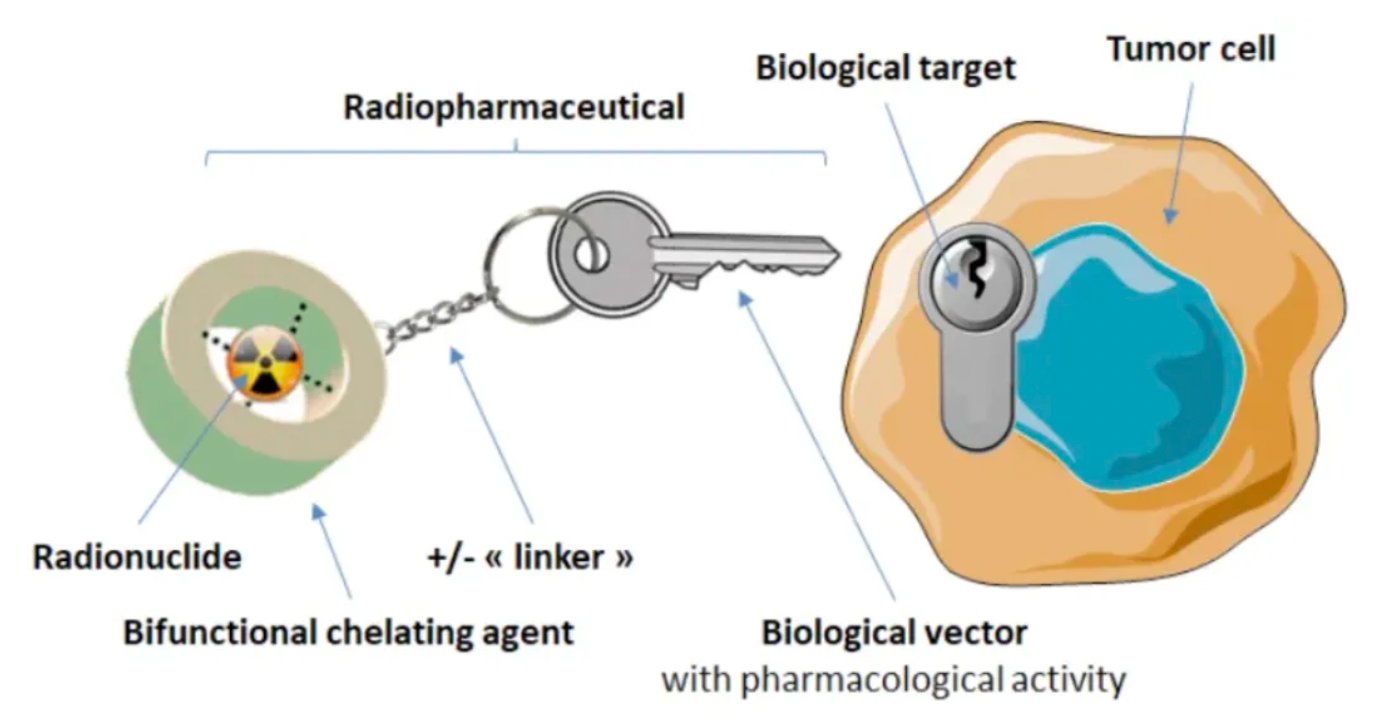
There are various specific ligands which bind to cancer cells, with antibodies, peptides, and small molecules being the most common. These can recognize markers or biochemical properties on the surface of cancer cells that are preferentially or overexpressed on these cells. When the ligand binds to the cell surface, it carries the attached radioactive isotope close to the cancer cells, initiating DNA damage and ultimately leading to cell death. The decay caused by the ligand-cell binding also releases energy that can damage the DNA of nearby cells, thereby producing what is known as the "bystander effect."
The radioactive isotopes used in RLTs can emit either α or β particles, each with distinct characteristics and potential applications. α-emitting isotopes release high-energy α particles during decay, which have large volume and mass and expend their energy within a short distance; whereas β-emitting isotopes release β particles that have deeper penetration but cause less clinically relevant damage to DNA. The shorter penetration depth of α particles is considered potentially less concerning regarding safety and side effects because α particles may have a lower "bystander effect" on non-cancerous cells. Due to the higher efficacy of α-emitting isotopes and reduced off-target effects, there is growing interest in using α-emitting radioactive isotopes (especially actinium and lead) in research and development. However, owing to production and procurement challenges, as well as the familiarity of medical staff with β-emitters, RLTs approved to date still utilize β-emitting isotopes.
Current research and development in radioligand therapy (RLT) encompasses a wide range of tumor types, including breast cancer, lung cancer, and hematological malignancies, which have substantial potential patient populations.
The research involves fifteen different isotopes. In addition to extensive development of monotherapies, combination therapies with RLT are also being explored. When used in conjunction with other treatments, such as chemotherapy and immunooncology (IO) therapies, they can induce additional DNA damage and biochemical cellular signaling cascades, potentially leading to stronger therapeutic effects. Early studies indicate that when PD-L1 inhibitors are combined with RLT, a synergistic cell-killing effect is observed. DDR (DNA Damage Response) inhibitors, like PARP inhibitors, which interfere with DNA break repair, allow cells to accumulate substantial DNA damage and may enhance the cytotoxic effects of RLT. In addition, there are ongoing trials of RLT in combination with other classes of drugs, such as CDK4/6 inhibitors, mTOR inhibitors, and DNA methyltransferase inhibitors, which can also lead to tumor cell death.
Due to optimistic market expectations for growth potential, the field of radioligand therapy (RLT) has attracted considerable transaction activity in recent years. In 2023, venture capital transactions in this sector increased by approximately 550%, exceeding $400 million, compared to about $65 million in 2017. The new entrants in the industry include both large well-known pharmaceutical companies and small specialty biotech firms. According to an analysis of the RLT research and development pipeline, by the end of 2022, over 60 participants had entered this sector. With these therapies achieving greater clinical and commercial success, this number is expected to continue to rise.
Any manufacturer wishing to enter and succeed in the RLT field must deeply understand the fundamental differences between RLT and traditional therapies. RLT development requires interdisciplinary expertise, including radiochemistry, pharmacology, medical physics, radionuclide imaging, and dosimetry—areas that are generally new to most manufacturers. Therefore, possessing the appropriate technical capabilities and functional strengths, as well as a corporate culture familiar with the unique challenges in the development and commercialization of RLT, are crucial factors for success. Manufacturers need to grasp skills in the synthesis of radiopharmaceuticals, pharmacokinetics, medical dosimetry, and nuclear medical imaging, while also establishing organizational structures and mindsets tailored to the unique requirements of RLT, to effectively compete in this field.
Production of radioligand therapy (RLT) necessitates a complex supply chain, involving the production of isotopes, generation of RLT, and transportation to the administration sites. In the product life cycle of RLT, manufacturing and distribution are key steps, which entail many unique complexities, many of them related to the short half-life of radioactive isotopes. RLTs, with their short shelf-life, require “just-in-time” manufacturing processes, ideally in a decentralized manner close to where the RLTs are used. Centers like academic medical institutions that extensively use RLT and radiologic imaging tools may have their own internal radiopharmacy to prepare RLTs for final administration.
Translation of RadioLigand Therapy (RLT) production, manufacturing, and distribution process:
·Raw Material Production: Raw materials (e.g., Lutetium-176) are sent to companies with nuclear reactors.
·Nuclear Reactor: The raw materials undergo a nuclear fission reaction within the reactor to produce radioactive isotopes.
·Radioactive Isotopes: After QC checks for radioactivity levels and packaging, radioactive isotopes are boxed.
·Dispensation and Transport of Radioactive Isotopes: Radioactive isotopes are sent to contract manufacturers.
·Drug Manufacturing Facilities: 5a. In-house Manufacturing Facilities: Companies like Novartis possess internal facilities capable of large-scale production of RLT. 5b. Contract Manufacturers: When a company employs a contract manufacturer, radioactive isotopes are often sent directly to them for handling all procurement, QC, and distribution.
·Manufacturer's QC: Upon completion of RLT production by the Contract Manufacturing Organization (CMO), the product may then be sent back to the manufacturer for quality control and begin shipping.
·Production and Distribution: After RLT manufacture, some companies or CMOs might send products to logistics companies for transportation.
·Logistics Transportation: 8a. Logistics Companies: Distributors transport RLT to nuclear pharmacies (9) and administration sites (10). 8b. Nuclear Pharmacies: Companies with logistics needs or CMOs can send RLT directly to nuclear pharmacies or administration sites, depending on the site’s capability. 8c. Administration Sites: RLT is sent to administration sites.
·Nuclear Pharmacy: The nuclear pharmacy prepares the medication for administration (ensuring radioactivity levels and delivering RLT to administration sites).
·Administration Sites: Administration takes place in Nuclear Medicine Services or radiation oncology clinics, considering infrastructure requirements and the availability of authorized users.
·Patients: RLT is administered to patients.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!