Nutcracker Therapeutics Highlights Enhanced Efficacy of NTX-472 for B Cell Lymphoma at 2024 ASCO
Nutcracker Therapeutics, Inc., a biotech firm focused on creating groundbreaking RNA treatments with its unique technology platform, has recently showcased a poster on NTX-472, an emerging preclinical drug candidate for B cell lymphoma, at the 2024 American Society of Clinical Oncology Annual Meeting in Chicago.
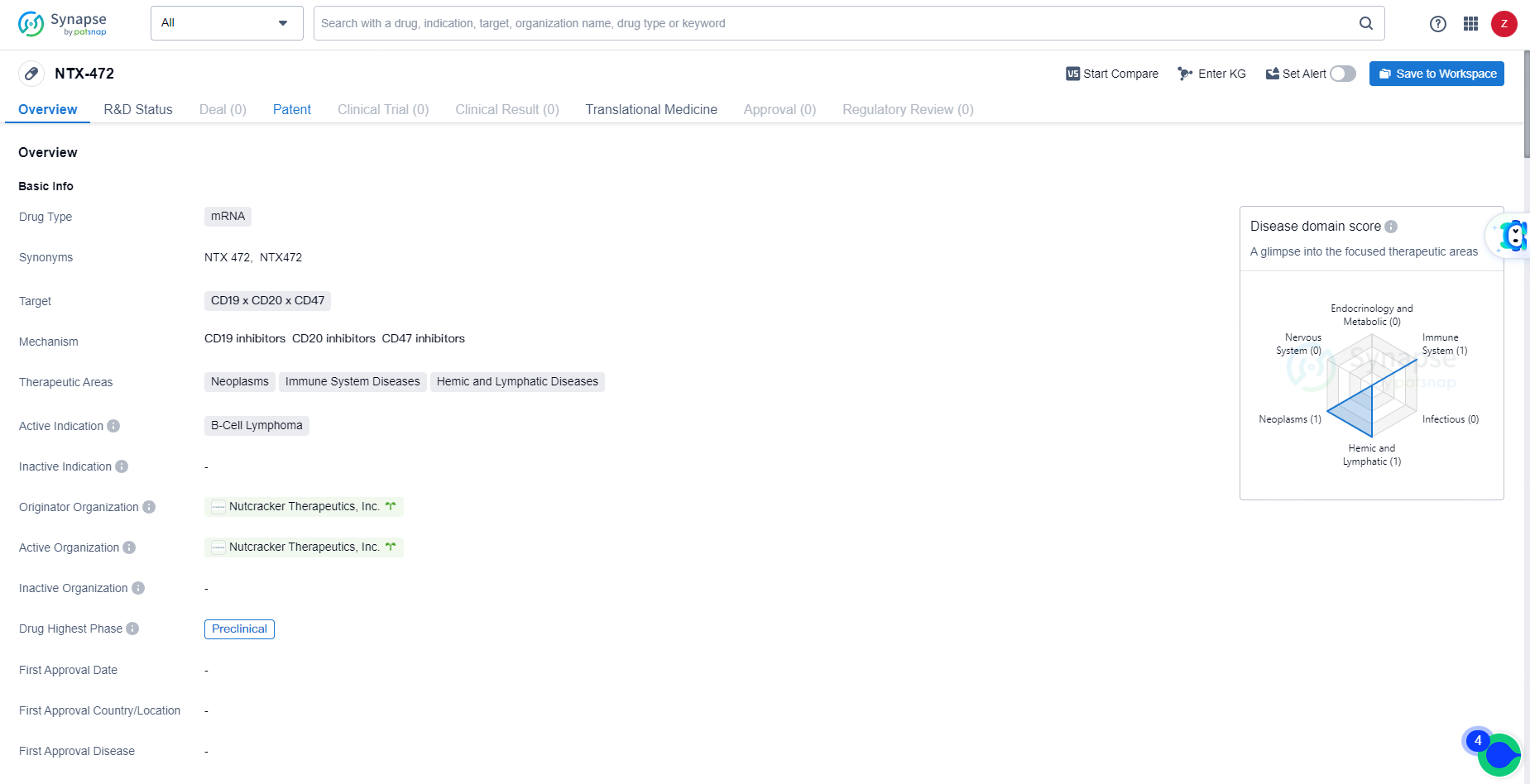
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Monoclonal antibody immunotherapies pose a promising treatment strategy for B cell lymphoma. However, these therapies often focus on a single tumor antigen, such as CD19 or CD20, which can lead to selective pressures on tumors. This selective targeting frequently causes cancer cells to down-regulate these specific antigens, resulting in the development of cold tumors that evade immune detection. To address this issue, multispecific antibodies, which simultaneously recognize multiple antigens, offer a potential solution.
Researchers at Nutcracker Therapeutics developed a series of molecules aimed at targeting CD20, CD19, and CD47 concurrently, allowing for a comparison with existing monoclonal antibody immunotherapies like rituximab and tafasitamab used for B cell lymphoma. Among these molecules, one demonstrated enhanced tumor cell eradication and B cell depletion in vitro, leading to the establishment of the NTX-472 program.
Using advanced tools like CodonCrackerTM software and the Nutcracker® Manufacturing Unit, Nutcracker Therapeutics' scientists evaluated NTX-472 in vivo. The results showed swift B cell depletion without observable binding to red blood cells in cynomolgus monkeys.
“Our achievement as one of the pioneering RNA therapeutics companies to develop a multispecific antibody fills us with pride,” remarked Samuel Deutsch, Ph.D., Chief Scientific Officer. “The NTX-472 data exemplifies the capabilities of Nutcracker Therapeutics’ platform, enabling our researchers to design complex molecules in large numbers and identify the most promising drug candidates. We aim to advance NTX-472 into a trispecific immunotherapy featuring a distinctive therapeutic and safety profile.”
In previous presentations, Nutcracker Therapeutics shared preclinical findings on NTX-471, an mRNA therapeutic candidate focusing on CD47. In contrast to the multispecific strategy of NTX-472, NTX-471 is designed to encode a multivalent antibody, enhancing specificity through strong binding to target cancer cells. At SITC 2023, Nutcracker Therapeutics highlighted NTX-471's cytotoxic efficacy comparable to existing anti-CD47 treatments, with minimal to no red blood cell binding. Further details on NTX-471 can be accessed in this press release.
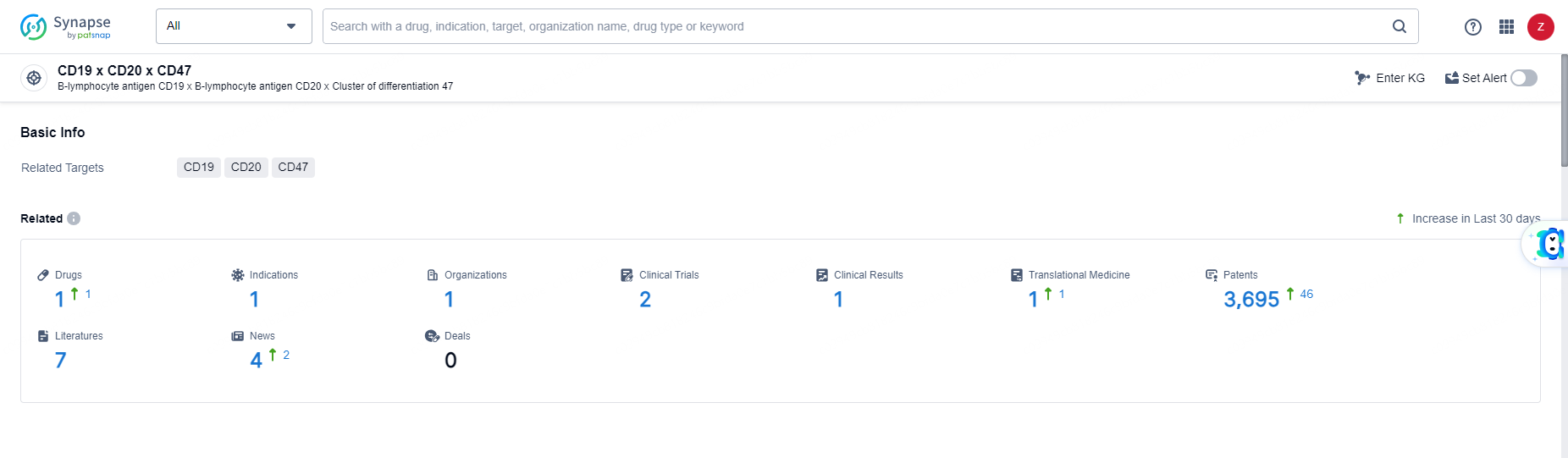
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 11, 2024, there are 1 investigational drugs for the CD19 and CD20 and CD47 target, including 1 indications, 1 R&D institutions involved, with related clinical trials reaching 2, and as many as 3695 patents.
NTX-472 targets CD19, CD20, and CD47 and is currently in the preclinical phase of development. Further clinical data and trials will be necessary to determine the potential of NTX-472 as a treatment for B-Cell Lymphoma.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!