Accutar Reveals Phase 1 Results for AC699 in ER+/HER2- Breast Cancer at ASCO 2024
Accutar Biotechnology, Inc., a biotech firm specializing in AI-driven drug discovery, has shared findings from an ongoing Phase 1 clinical trial of AC699 as a monotherapy for patients with locally advanced or metastatic ER-positive / HER2-negative breast cancer. These results will be showcased in a poster discussion at the upcoming American Society of Clinical Oncology Annual Meeting in Chicago, IL, scheduled for June 1, 2024.
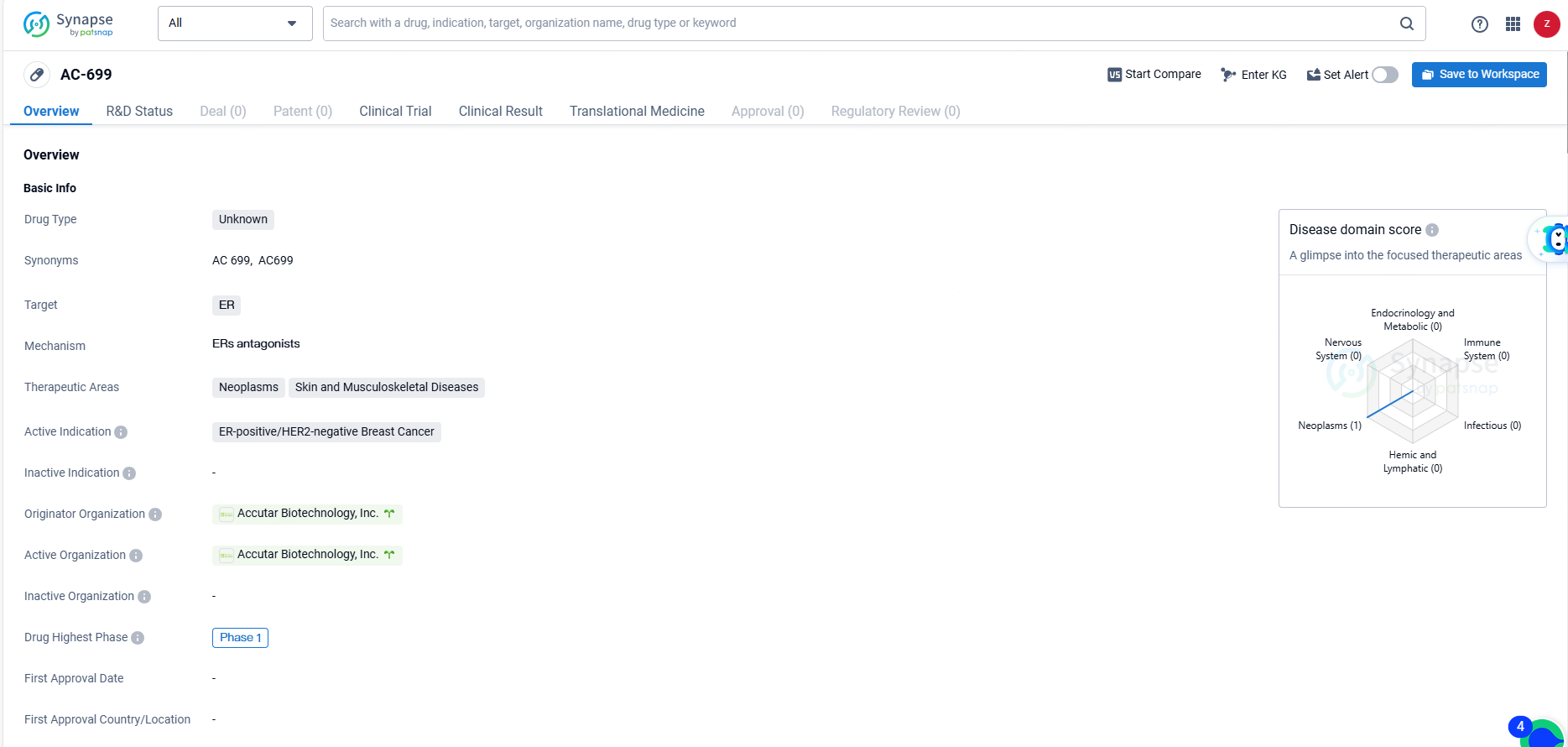
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
AC699 is an effective and selective orally bioavailable chimeric degrader targeting the estrogen receptor (ER) α. It represents a potential novel treatment option for breast cancer by employing a unique mechanism of action, which differs from fulvestrant and other novel SERDs, and shows enhanced ERα degradation as indicated by preclinical data.
AC699 is currently under evaluation in an ongoing Phase 1 clinical trial as monotherapy for patients with ER-positive / HER2-negative locally advanced or metastatic breast cancer. The primary goals of the study are to assess the safety and tolerability of AC699. Secondary and exploratory aims involve the study of pharmacokinetics, initial efficacy, and pharmacodynamic properties. This clinical trial follows a 3+3 dose-escalation design, administering AC699 orally once daily at doses of 100, 200, 300, 400, and 600 mg.
"We are very encouraged by the remarkable safety and efficacy that AC699 has shown in Phase 1, indicating its best-in-class potential, particularly for patients with ESR1 mutations," stated Jie Fan, Ph.D., the Chief Executive Officer of Accutar Biotechnology, Inc. "We eagerly anticipate completing the dose escalation phase and commencing Phase 2 soon. We believe that the oral administration of AC699 and its distinct mechanism of action, compared to fulvestrant and other novel SERDs, could offer a new, safe, and efficient treatment option for this patient group," Jie Fan added.
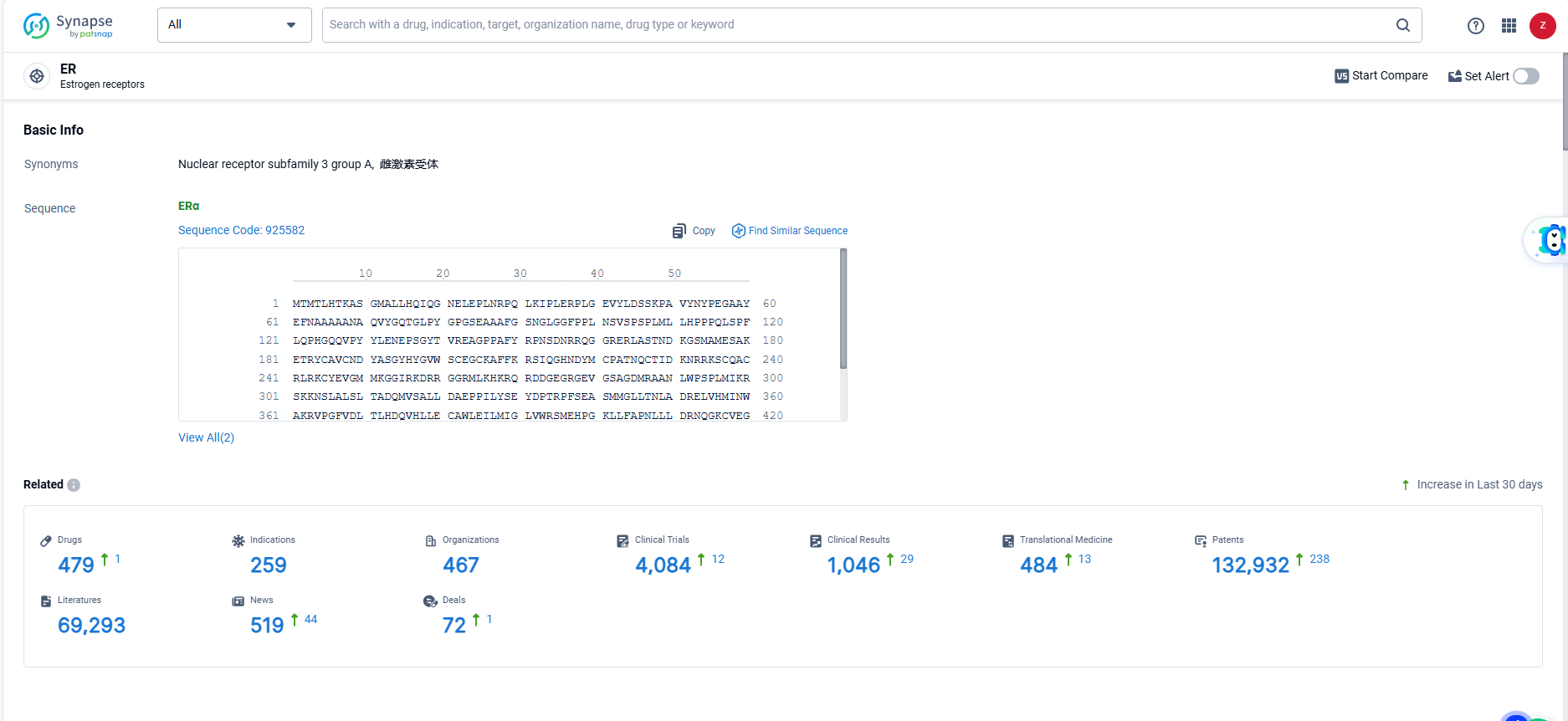
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 7, 2024, there are 479 investigational drugs for the ER targets, including 259 indications, 467 R&D institutions involved, with related clinical trials reaching 4084, and as many as 132932 patents.
AC-699 is a drug of unknown type that targets the ER and is being developed for the treatment of ER-positive/HER2-negative breast cancer, as well as neoplasms, skin, and musculoskeletal diseases. It is being developed by Accutar Biotechnology, Inc. and has reached Phase 1 of development globally.