OKYO Pharma's OK-101 Excels in Phase 2 Trial with Significant Dry Eye Relief
OKYO Pharma Limited, which operates within a market worth several billions, and is engaged in addressing front-of-the-eye conditions such as neuropathic corneal pain—a condition that causes discomfort yet lacks an approved treatment by the FDA—has disclosed further significant insights from the examination of clinical data obtained from a study comprising 240 participants. This Phase 2 study was organized as a randomized, double-blind, placebo-controlled trial, which aimed to assess the safety and effectiveness of the OK-101 eye drop solution in individuals suffering from Dry Eye Disease (DED).
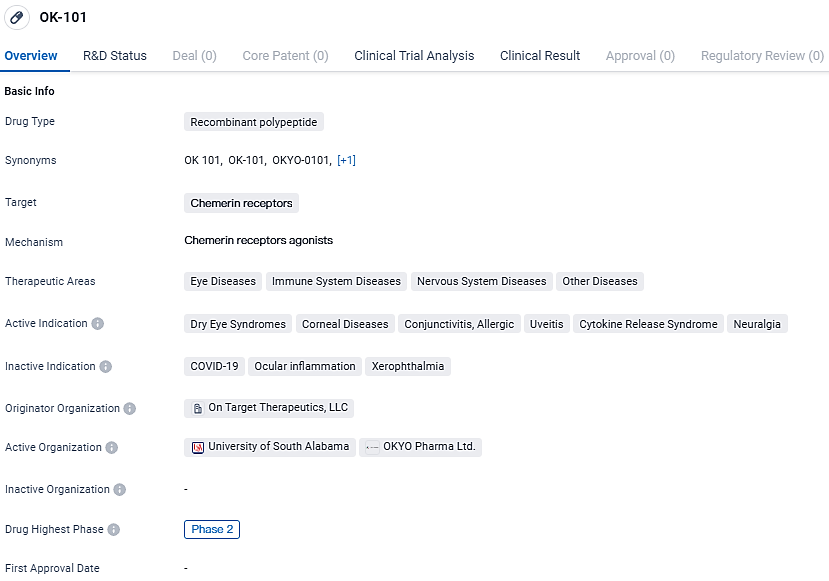
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The enterprise had previously disclosed that there were notable enhancements in total conjunctival staining, gauged by the Ora Calibra© Staining Scale on Day 29, along with a decrease in sensations of burning/stinging and occurrences of blurred vision, as rated on a visual analogue scale with burning/stinging being apparent on Day 15 and blurred vision on Day 29. The undertaking of this Phase 2 study was by the Company's CRO collaborator, Ora Inc.
Within this update, the enterprise is sharing further findings related to OK-101, including prolonged effects on conjunctival staining noted at Day 85 which underscores the persisting efficacy on this particular clinical sign. Also noted were the enduring reductions in the issues of burning/stinging and blurred vision over the duration of the study.
Dr. Jay Pepose, M.D., Ph.D., the founder and the Chief Medical Officer of Pepose Vision Institute as well as Clinical Ophthalmology Professor at Washington University School of Medicine in St. Louis, remarked on the substantial influence of OK-101. He highlighted its effectiveness in promptly and lastingly increasing tear film breakup time; this is vital for a large number of individuals dealing with dry eye syndrome who suffer from reduced blinking because of prolonged exposure to digital screens, reading, or driving. Dr. Pepose continued to explain that the stabilization of the tear film is congruent with the mitigation of several symptoms associated with dry eye, such as the impairment of clear vision. A quick deterioration of the tear film is a characteristic found in all variants of dry eye pathology, inclusive of aqueous deficit, evaporative types, and combined conditions.
OK-101, a lipid-conjugated chemerin peptide agonist targeting the ChemR23 G-protein coupled receptor localized on ocular immune cells that orchestrate the inflammatory response, has been observed to yield anti-inflammatory and analgesic effectiveness in both dry eye syndrome and corneal neuropathic pain in murine models. OK-101's design includes a lipid anchor to promote retention within the eye, aiming to counteract drug dilution.
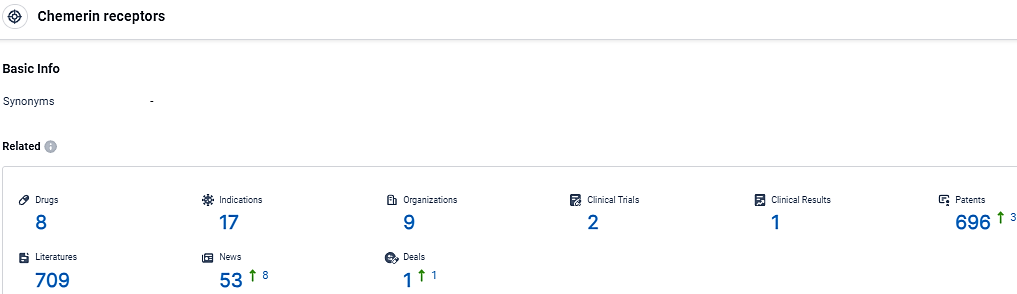
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 25 2024, there are 8 investigational drugs for the Chemerin receptors target, including 17 indications, 9 R&D institutions involved, with related clinical trials reaching 2, and as many as 696 patents.
OK-101 targets chemerin receptors and has a broad range of therapeutic areas, including eye diseases, immune system diseases, nervous system diseases, and other diseases. With its current status in Phase 2, OK-101 shows promise in addressing conditions such as dry eye syndromes, corneal diseases, conjunctivitis, uveitis, cytokine release syndrome, and neuralgia.