Opsidio began dosing the first patient in a Phase 2a trial of OpSCF for moderate to severe eczema treatment
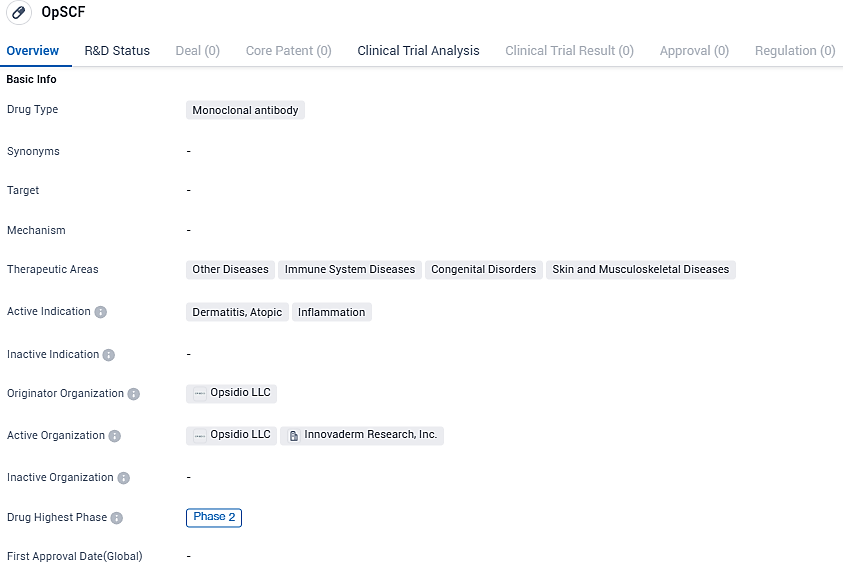
Opsidio, LLC, a biotech organization actively engaged in the development of novel treatments aimed at addressing inflammation-related health conditions, has confirmed commencement of treatment with their first subject in the early Phase 2a study. This clinical trial is evaluating the therapeutic effects of their groundbreaking monoclonal antibody, known as OpSCF, which targets the stem cell factor. This study focuses on individuals who are experiencing moderate to severe forms of atopic dermatitis. Opsidio has entered into a partnership with the pharmaceutical giant, AbbVie, to facilitate the advancement of OpSCF.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Opsidio's CEO, Dr. Martin Phillips, is thrilled to share the launch of our Phase 2a clinical trial targeting atopic dermatitis, a pivotal move in our ongoing commitment to enhance the wellbeing of individuals battling persistent inflammatory disorders.
The actively recruiting OpSCF-201 trial is a placebo-controlled, double-masked, randomized exploratory Phase 2a trial designed to assess the therapeutic effect and safety profile of OpSCF in adult subjects suffering from moderate to severe atopic dermatitis. A key measure of success will be the variation in Eczema Area and Severity Index (EASI) scores from the study's outset, evaluated at the 16-week mark.
The trial also aims to measure other important outcomes, such as tolerability and safety, as well as improvements in itch intensity, the extent of body surface area affected, and overall patient quality of life. A noteworthy aspect of OpSCF-201 is its 40-week open label extension phase, ensuring that all participants may access OpSCF throughout their study participation duration.
In its earlier phase 1a/b clinical trial, which included 115 individuals, OpSCF demonstrated a favorable safety profile and was generally well-tolerated, whether administered as a single dose or in multiple ascending doses amongst a cohort of healthy volunteers.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
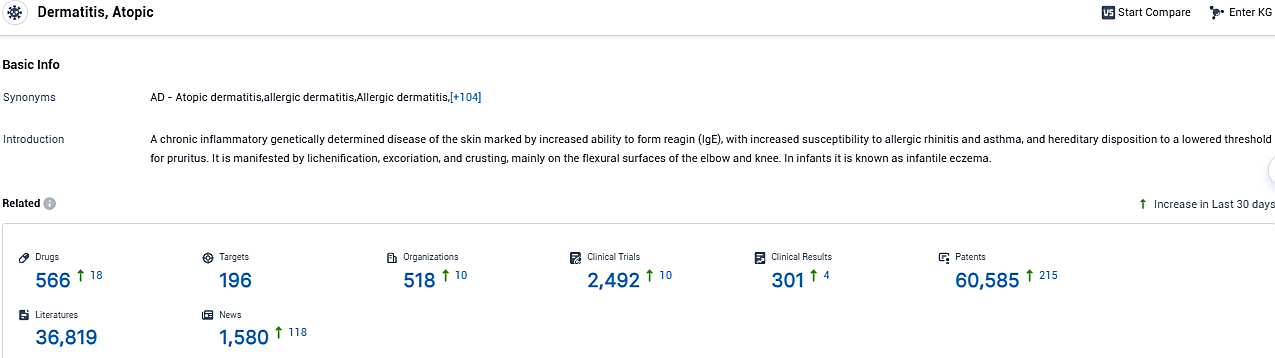
According to the data provided by the Synapse Database, As of December 7, 2023, there are 566 investigational drugs for atopic dermatitis, including 196 targets, 518 R&D institutions involved, with related clinical trials reaching 2492, and as many as 60585 patents.
OpSCF is a monoclonal antibody that targets stem cell factor which plays a crucial role in the inflammatory pathway that leads to atopic dermatitis. By targeting a specific isoform of stem cell factor that drives inflammation, we hope to offer patients a more effective and durable treatment option, while avoiding the negative effects of broad stem cell factor inhibition.