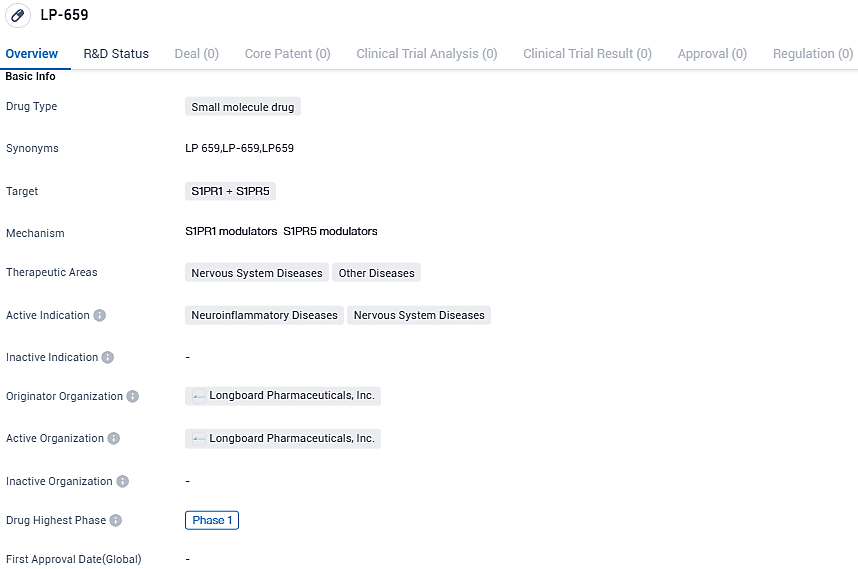
Longboard Pharmaceuticals Starts Phase 1 Human Trial for LP659 with Healthy Adult Participants
Longboard Pharmaceuticals, Inc., an enterprise deeply engaged in the clinical realm and dedicated to creating groundbreaking therapeutic solutions for conditions affecting the nervous system, has recently declared the commencement of a Phase 1 trial. This study, featuring a randomized, double-blind, and placebo-controlled design for Single Ascending Dose (SAD), will evaluate the investigational drug LP659.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
LP659 is a novel oral compound that targets the central nervous system, designed as an advanced modulator for the subtypes 1 and 5 sphingosine-1-phosphate (S1P) receptors, currently being developed for the targeted management of uncommon neuroinflammatory disorders.
Kevin R. Lind, who holds the positions of President and CEO at Longboard, expressed enthusiasm about augmenting their leading clinical-stage portfolio with LP659. Longboard was established on the backs of Arena's proven expertise in the realms of molecular discovery and development. This expertise is encapsulated in their distinct methods for pharmacokinetics, pharmacodynamics, and active receptor engagement. Lind announced that updates regarding initial Phase 1 single-ascending dose (SAD) study results and further clinical strategies for LP659 will be released in the first two quarters of 2024.
Devised by the creators of etrasimod, LP659 aimed to forge a new generation of brain-penetrating S1P receptor modulators, ensuring the preservation of distinct characteristics, such as the omission of activity on the S1P2 and S1P3 receptor subtypes. These particular subtypes have previously been linked to significant negative effects.
The foremost goal of this Phase 1 study, which is organized in a randomized, double-blind, placebo-controlled format with single-ascending dose administration, is to meticulously assess the safety, tolerability, pharmacokinetic properties, and pharmacodynamic effects of LP659. This will involve enlisting up to 48 healthy adult participants.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
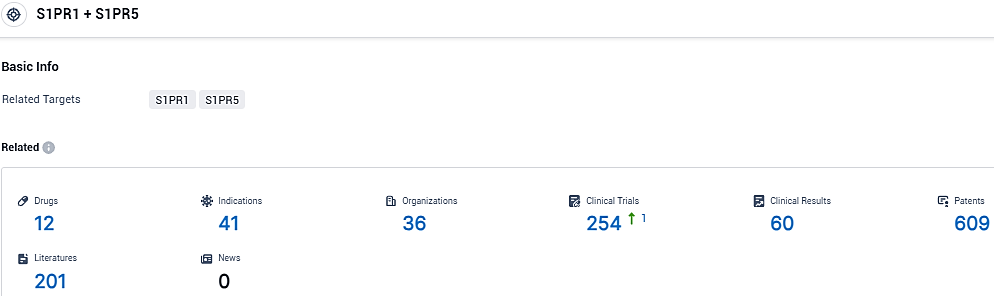
According to the data provided by the Synapse Database, As of December 6, 2023, there are 12 investigational drugs for the S1PR1 and S1PR5 target, including 41 indications, 36 R&D institutions involved, with related clinical trials reaching 254, and as many as 609 patents.
LP659 is an oral, centrally acting, next-generation S1P receptor subtypes 1 and 5 modulator in development for rare neuroinflammatory conditions. LP659’s potential selectivity and specificity could result in a superior profile in the clinic compared to drugs that may not fully engage the intended GPCR target, may cause off-target activity, or may be associated with other undesirable effects.