HOOKIPA Pharma reveals FDA's approval for their new investigative drug, HB-500, to treat HIV
HOOKIPA Pharma Inc., a firm involved in the creation of a novel category of immunotherapeutics via its unique arenavirus platform, has officially disclosed that the FDA has given the clearance for its IND application for HB-500, This is a new arenaviral therapeutic vaccine designed for HIV treatment.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
HOOKIPA is tasked with propelling the HIV project towards completion with a Phase 1b clinical trial. Post this, Gilead gains exclusive jurisdiction to carry on with the program's development.
"We're proud to announce our fourth active IND project at HOOKIPA, attesting to the wide-ranging potential of our arenavirus platform-encompassing several diseases and indications." said Joern Aldag, HOOKIPA's Chief Executive Officer. "Devising a strong and extensive T cell reaction capable of eliminating infected cells is key to combating HIV. Our groundbreaking arenaviral therapeutic vaccine holds potential to tackle the prevalent gap for a functional HIV cure."
Nature Partner Journals Vaccines recently publicized the co-preclinical analysis by HOOKIPA and Gilead, which acted as groundwork for the IND submission. The examinations published involved a simian immunodeficiency virus model, frequently applied as an HIV surrogate in preclinical settings. The data revealed that:
The arenaviral therapeutic vaccination was well tolerated, generating vigorous, reliable and lasting immune reactions in non-human primates; also, The arenaviral therapeutic vaccination considerably mitigated the SIV viral load and clinical symptoms in these animals in comparison to a placebo.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
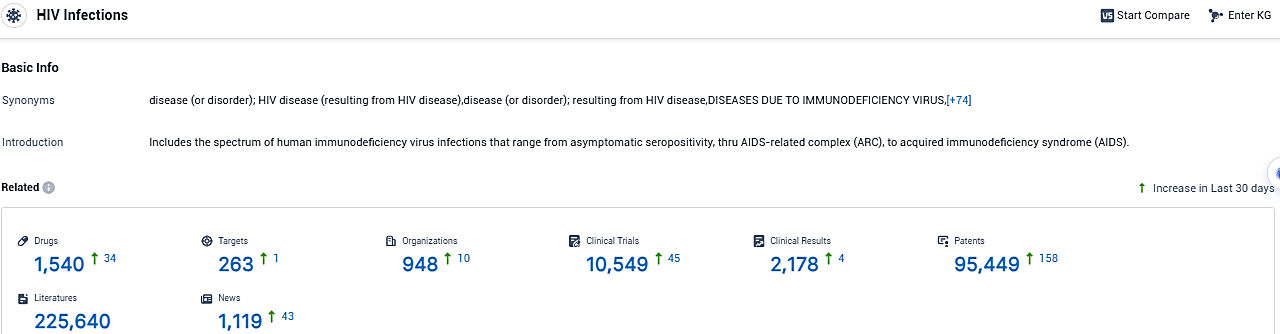
According to the data provided by the Synapse Database, As of November 28, 2023, there are 1540 investigational drugs for HIV Infections, including 263 targets, 948 R&D institutions involved, with related clinical trials reaching 10549, and as many as 95449 patents.
HB-500 is an alternating, 2-vector arenaviral therapeutic vaccine for the treatment of HIV. One vector is based on lymphocytic choriomeningitis virus as its arenaviral backbone; another vector is based on Pichinde virus Both encode the same HIV antigens. The alternating 2-vector approach is designed to further focus the immune response against the target antigen. HB-500 is one of two separate development programs in HOOKIPA’s collaboration and license agreement with Gilead.