Osimertinib mesylate: brief review of its R&D progress and the clinical result in 2023 ESMO
The 2023 ESMO Congress marked a pivotal moment with the disclosure of a clinical trial for Osimertinib mesylate in EGFR-mutated NSCLC patients, opening avenues for further increases in its clinical efficacy.
Osimertinib mesylate's R&D Progress
Osimertinib mesylate is a small molecule drug that is primarily used in the treatment of various types of cancers, particularly non-small cell lung cancer (NSCLC). It targets multiple mutations of the epidermal growth factor receptor (EGFR), including EGFR T790M, EGFR exon 21 L858R mutation, and EGFR exon 19 deletions. These mutations are commonly found in NSCLC patients and are associated with poor prognosis. 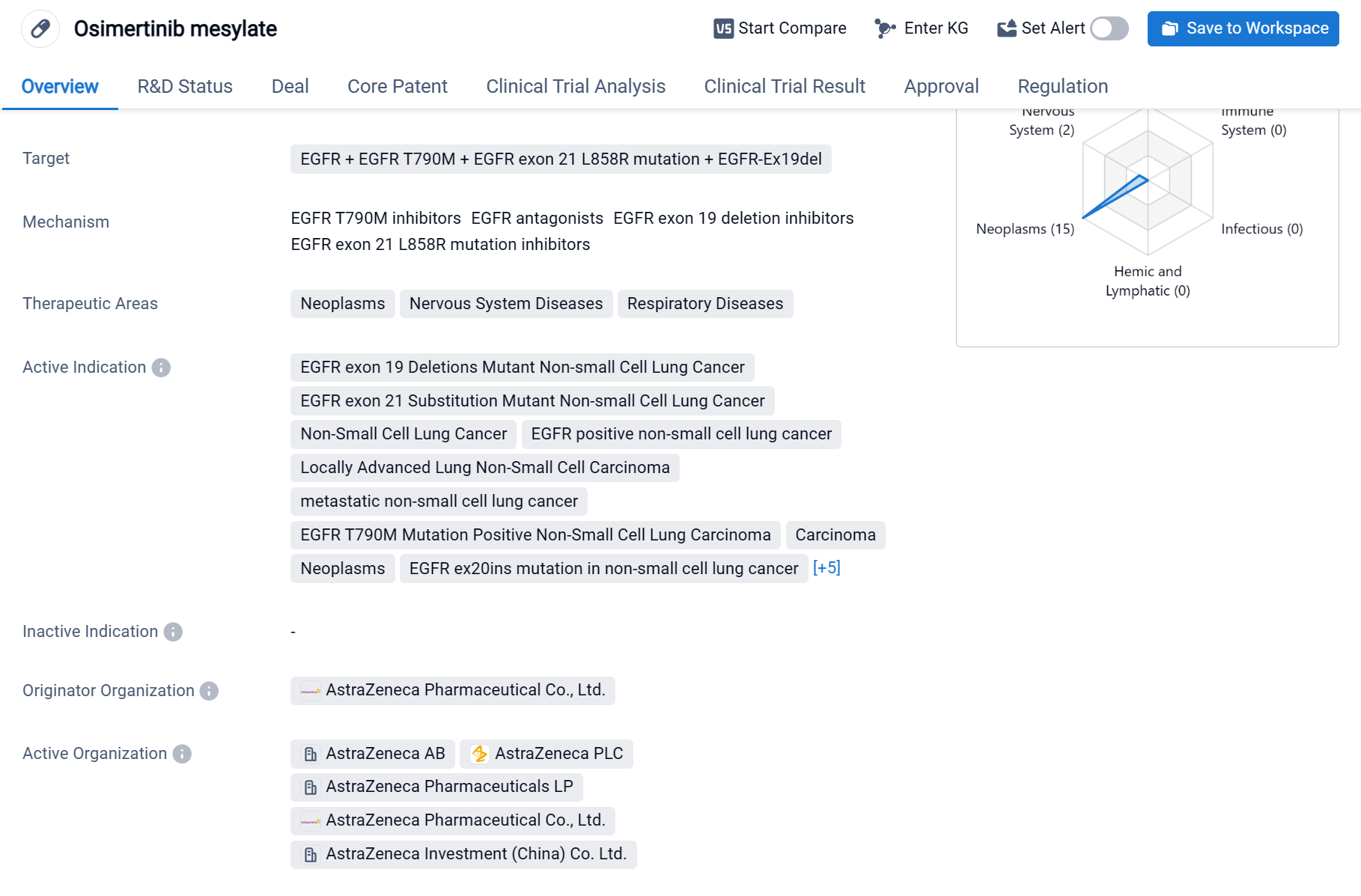
According to the Patsnap Synapse, Osimertinib mesylate was developed by AstraZeneca Pharmaceutical Co., Ltd., and it received its first approval in the United States in November 2015. It has since been approved in other countries as well. And the clinical trial areas for Osimertinib mesylate are primarily in the United States, China, and United Kingdom. The key indication is Non-Small Cell Lung Cancer. 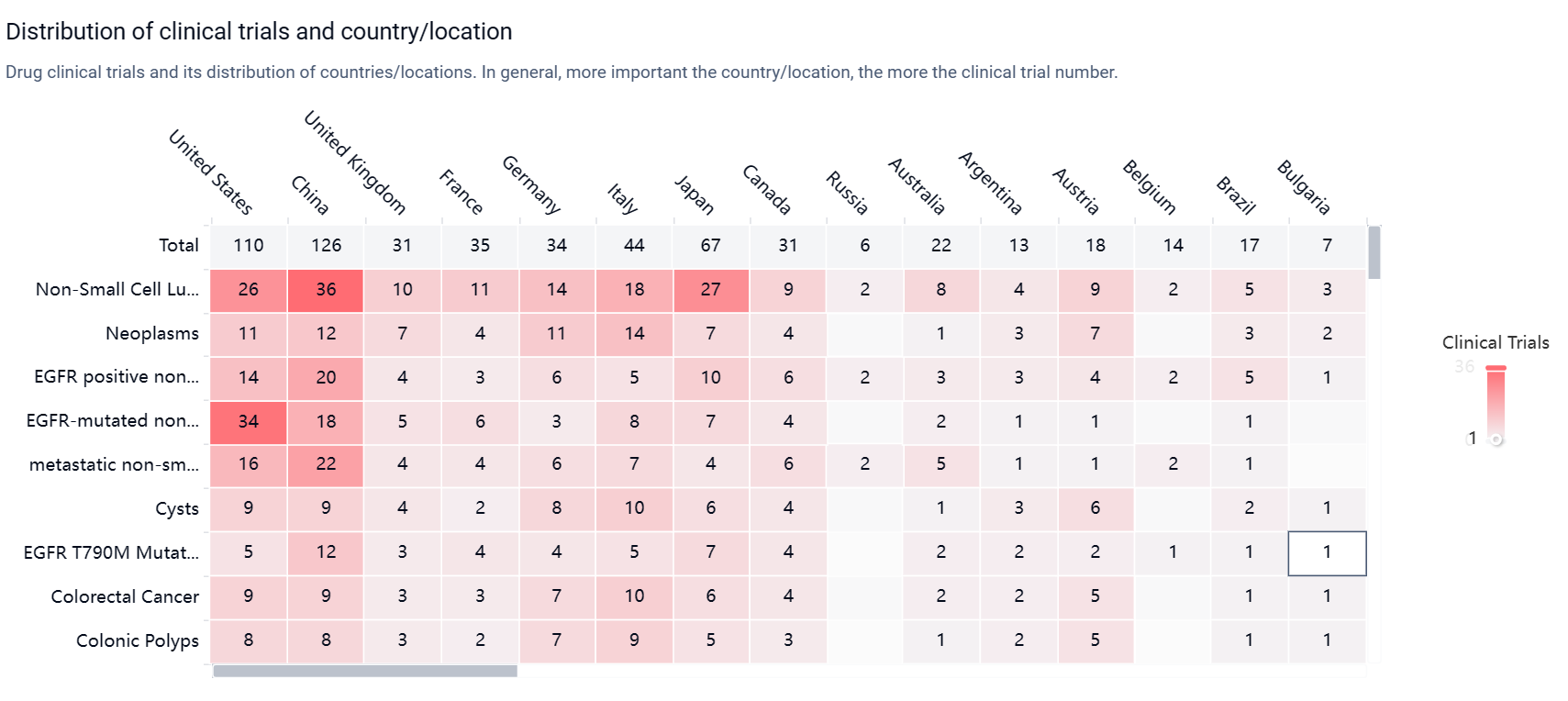
Detailed Clinical Outcome of Osimertinib mesylate
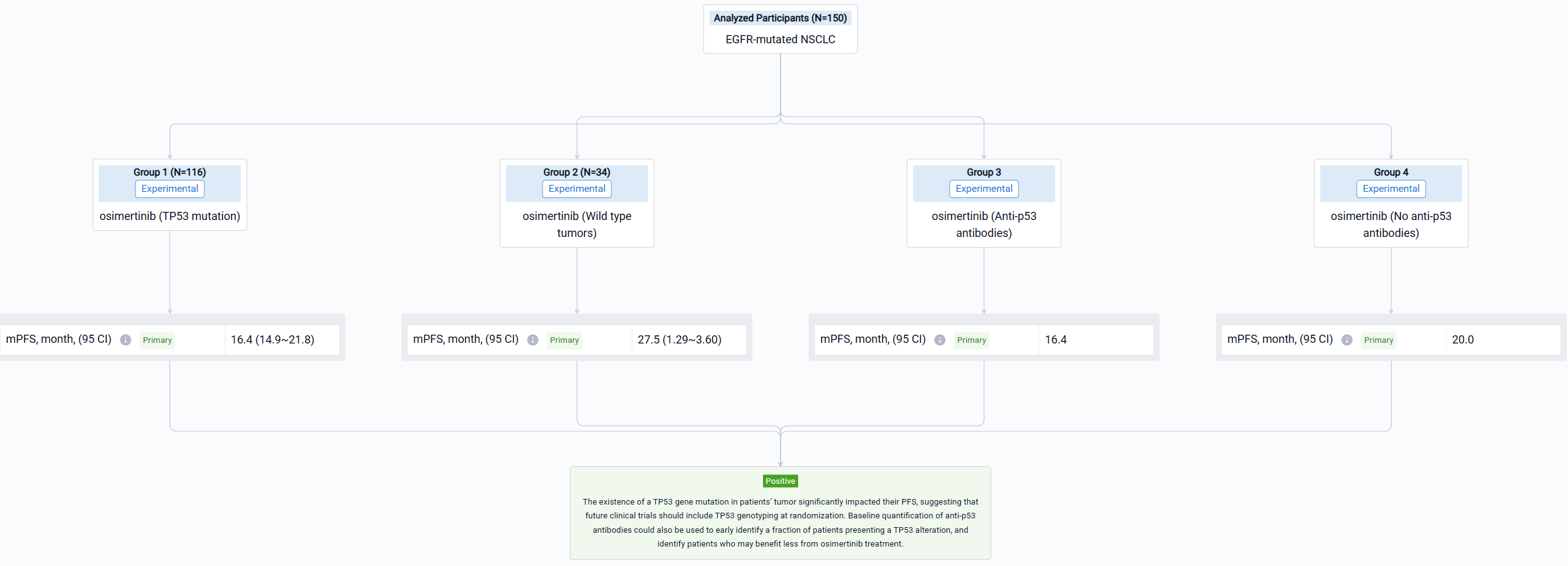
The multicentric phase II trial (NCT03865511) was designed to identify resistance mechanisms in treatment-naive EGFR-mutated NSCLC patients receiving Osimertinib and to be the first to evaluate the impact of TP53 mutations on the response to osimertinib.
In this study, the MELROSE trial enrolled 150 patients with EGFR-mutated (exon 19 deletion, n=88; L858R mutation, n=62) NSCLC. All patients received osimertinib. Tumor assessment was performed every 3 months, with brain and thoracoabdominal CT-scan. Tumor tissues collected at diagnosis were analyzed by next generation sequencing (QIAseq targeted DNA custom panel, QIAGEN). Anti-p53 antibodies were quantified in plasma using the automated Elecsys anti-p53 immunoassay (Cobas, Roche). 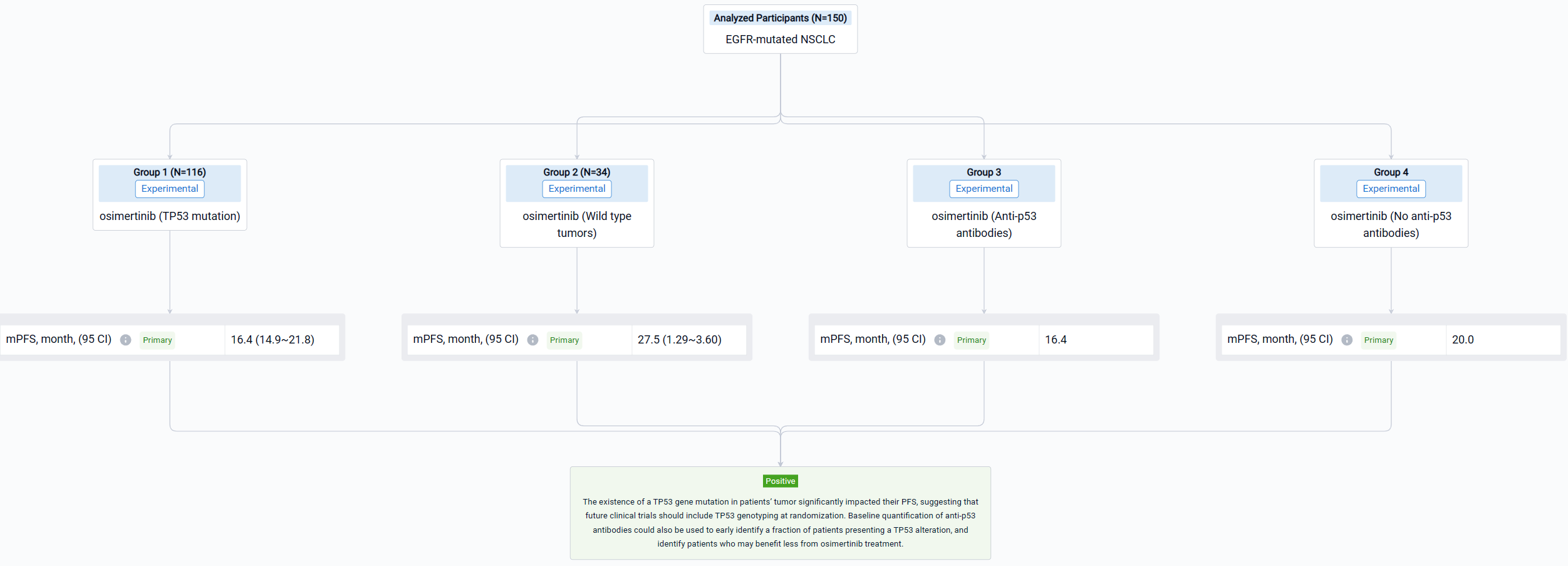
The result showed that Primary analysis of the whole cohort (data cutoff on April 4, 2023) showed a median PFS of 17.4 months (95% CI: 14.9-21.8). Tumor TP53 genotyping was successfully performed for 116 patients. TP53 mutations were detected in tumors of 74 patients (63.8%). Most mutations were present on exons 5 (n=14), 6 (n=13), 7 (n=12) and 8 (n=18). The presence of a TP53 mutation was associated with a reduced median PFS (16.4 months) as compared to wild type tumors (27.5 months; p=0.0034; HR 2.15; 95% CI: 1.29-3.60). Anti-p53 antibodies were detected at baseline in the plasma of 17.8% of the patients tested (26/146). The presence of antibodies was strongly associated with the existence of a TP53 mutation (p=0.001). Patients presenting detectable anti-p53 antibodies at baseline had a significantly lower median PFS (16.4 months) as compared to patients not presenting antibodies (20.0 months; p=0.034; HR 1.72; 95% CI: 1.04-2.83).
It can be concluded that the existence of a TP53 gene mutation in patients’ tumor significantly impacted their PFS, suggesting that future clinical trials should include TP53 genotyping at randomization. Baseline quantification of anti-p53 antibodies could also be used to early identify a fraction of patients presenting a TP53 alteration, and identify patients who may benefit less from osimertinib treatment.
How to Easily View the Clinical Results Using Synapse Database?
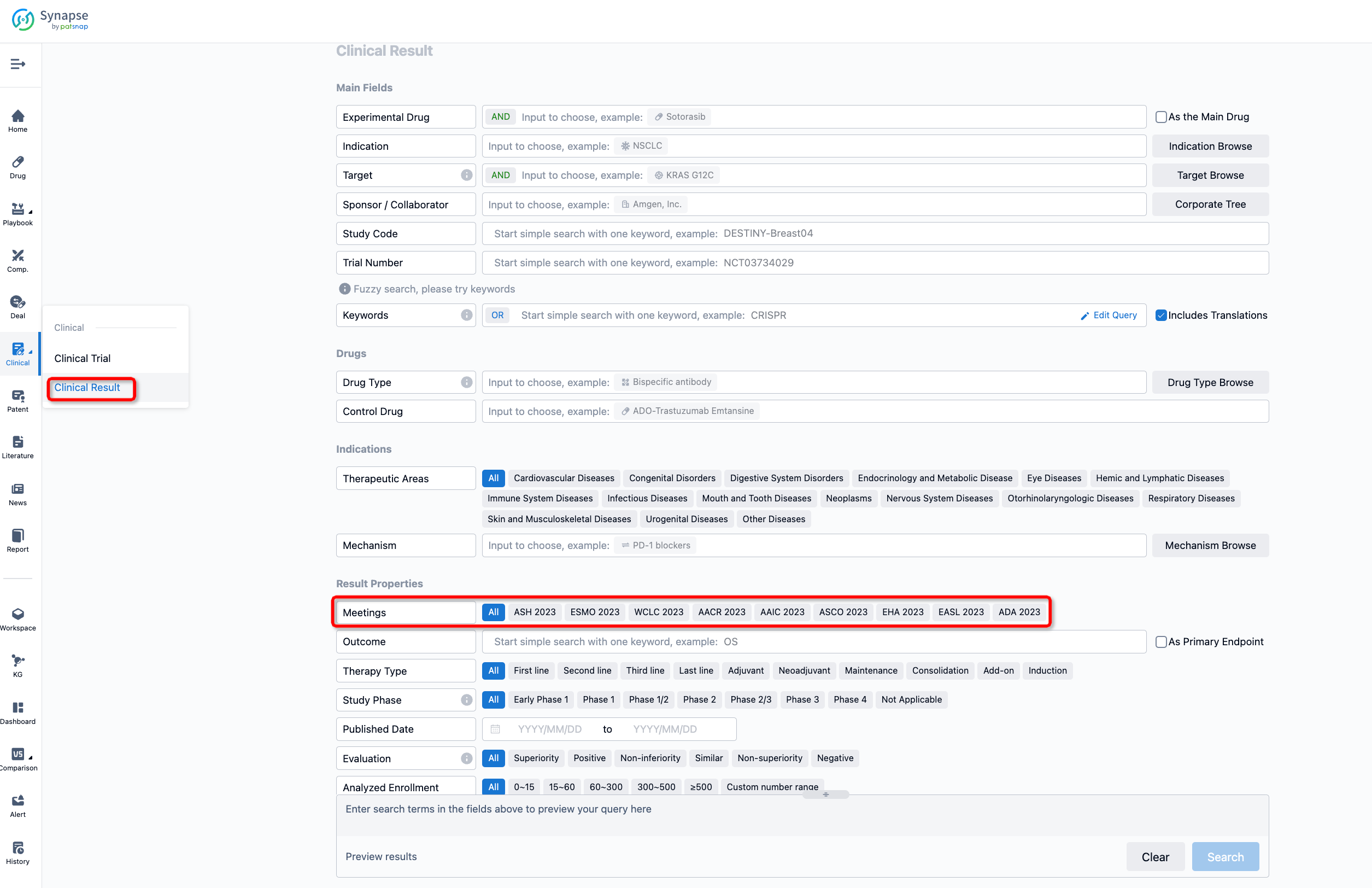
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
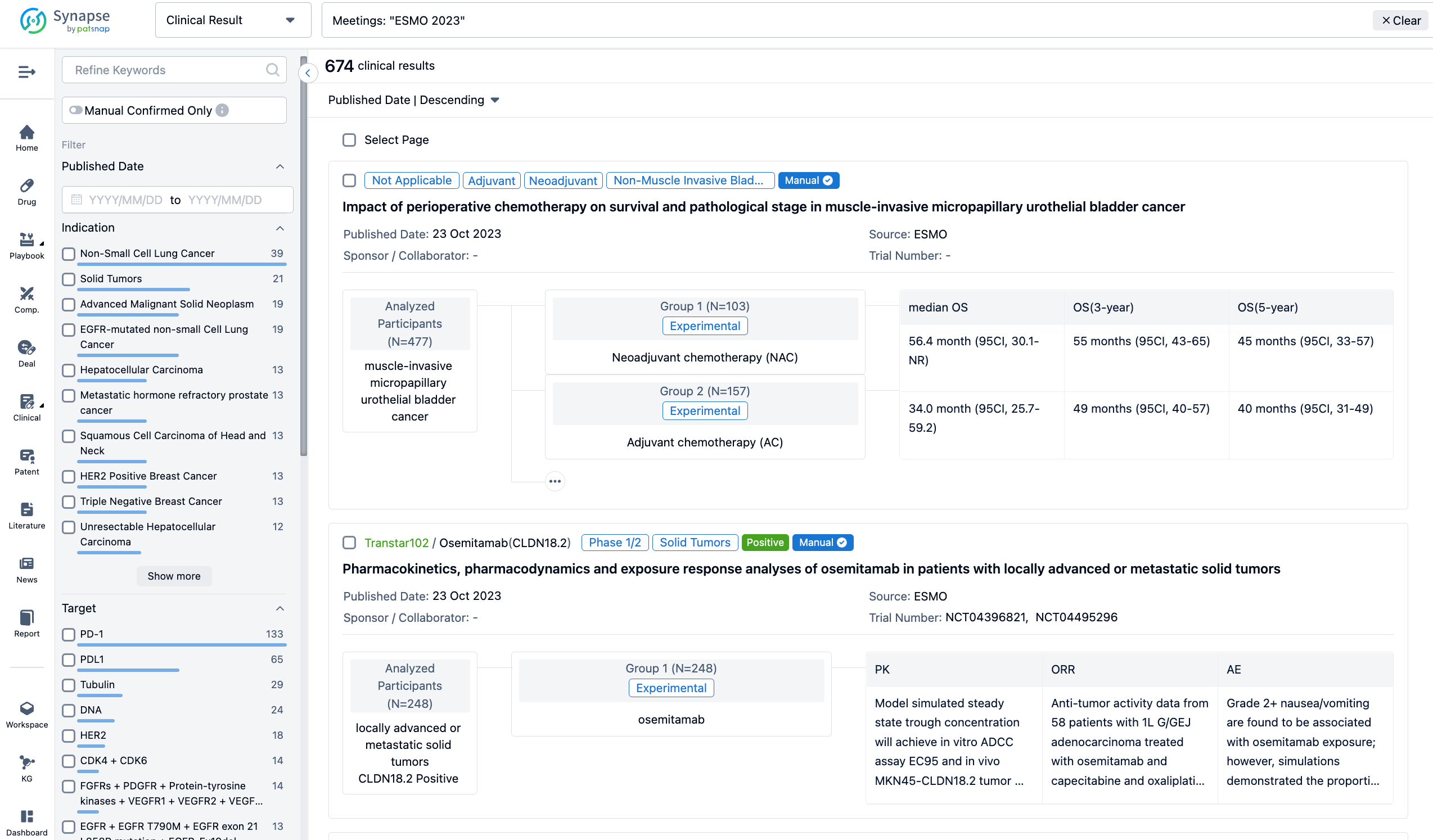
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.

In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
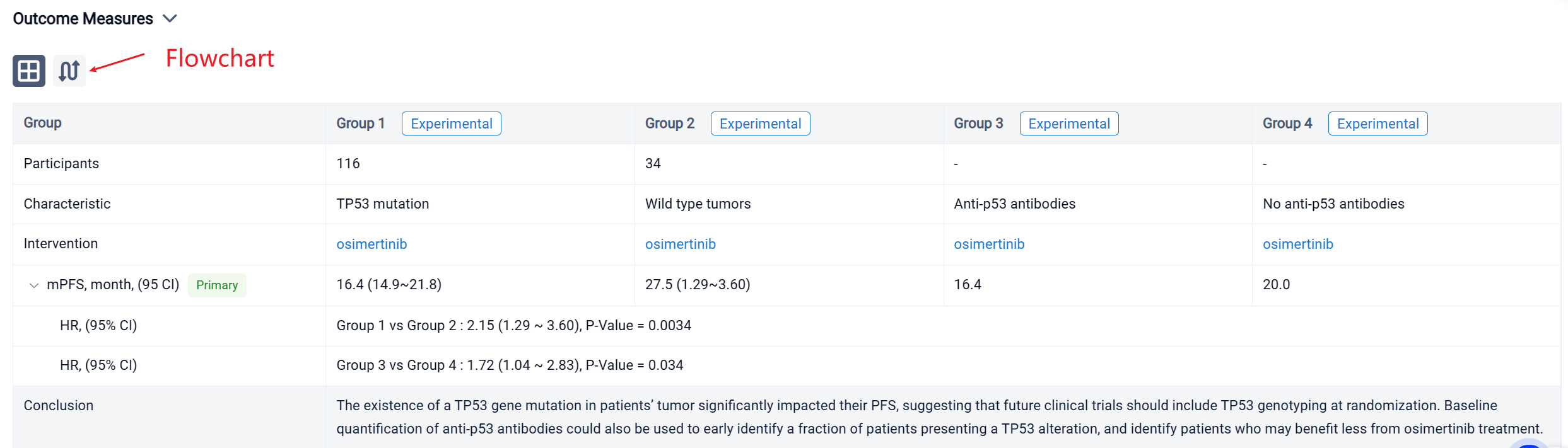
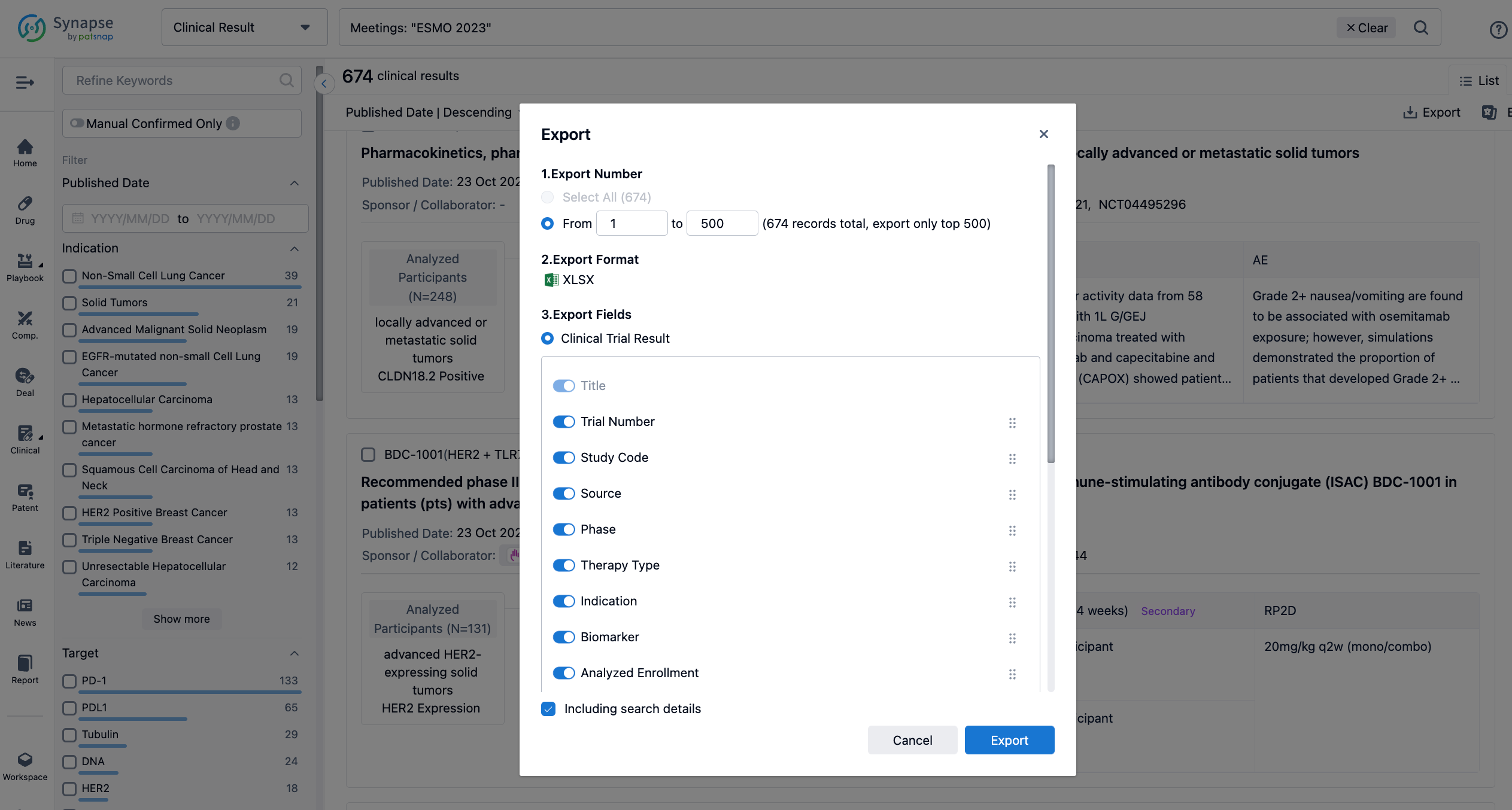
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!