Sale of GLP-1 Small Molecule to Eli Lilly, Chugai Pharmaceutical Discloses Latest KRAS Macrocyclic Patent
Last year belonged to the realm of Pfizer's COVID-19 small molecule treatments, while this year is dominated by GLP-1 injectable and oral therapies. The tremendous popularity of GLP-1 has propelled Novo Nordisk and Eli Lilly to reach market capitalizations of 500 and 600 billion US dollars respectively. Eli Lilly emerges as the biggest winner, not only does it have the GLP-1/GIP dual agonist Tirzepatide (brand name Mounjaro) approved in the US for weight reduction indications, but it also has a GLP-1 small molecule drug Orforglipron advancing in Phase III clinical trials.
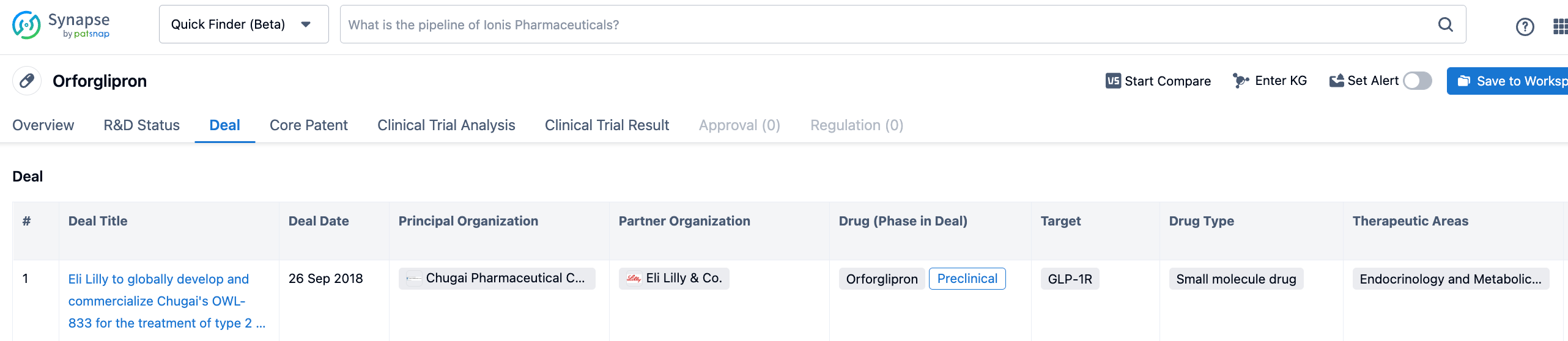
Upon searching the Synapse database for the keyword "Orforglipron," a transaction was identified. The transaction occurred on September 26, 2018, between Eli Lilly and Company and Chugai Pharmaceutical Co., Ltd. of Japan. According to the terms of the agreement, Eli Lilly acquired the global rights to develop and commercialize OWL833 (Orforglipron). In return, Chugai received an upfront payment of $50 million and is eligible to receive milestone payments upon the achievement of certain predetermined milestones. Furthermore, if Orforglipron is successfully commercialized, Chugai will also be entitled to royalties for patent usage. At the time, Orforglipron was in the preclinical stage. In comparison with the $185 million upfront payment that Chengyi Biologics received from AstraZeneca, Chugai indeed sold the asset at a relatively low price.
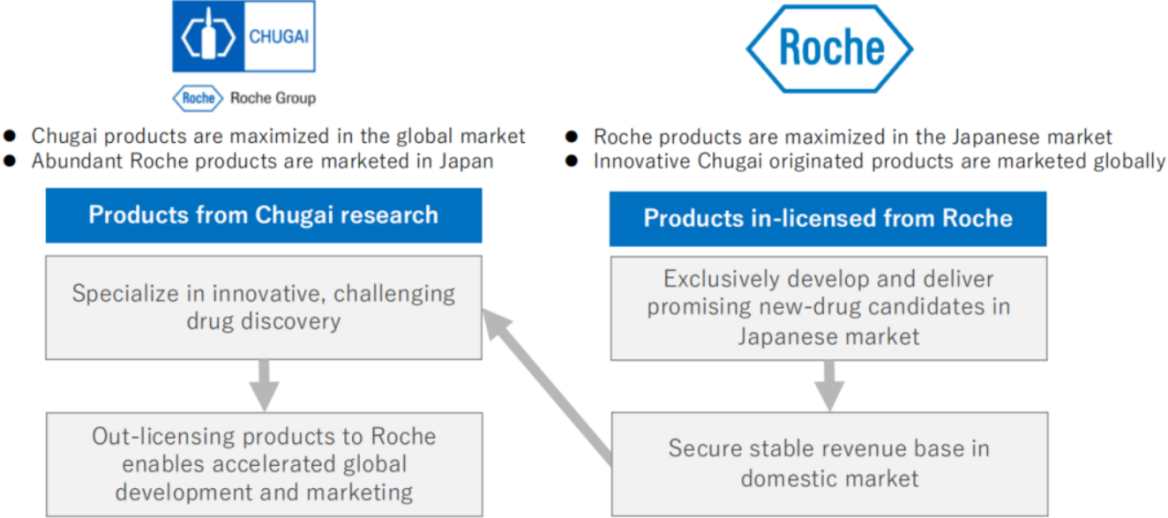
Chugai Pharmaceutical is a member of the Roche Group (with Roche currently holding 59.89% of Chugai's shares), yet it maintains autonomous and independent management, employing a unique business model focused on innovation that values individuality and diversity. Chugai's alliance with Roche provides it with the exclusive rights to market Roche's innovative pharmaceutical products in Japan, establishing a stable revenue foundation. This arrangement allows Chugai to concentrate its resources on unique and highly innovative technologies and drug discovery. Meanwhile, Chugai Pharmaceutical licenses out innovative drugs developed internally to Roche, which then supplies them to the global market. This enables Roche to sell pharmaceutical products innovated and developed by Chugai on a worldwide scale, thus creating a mutually beneficial relationship.
In addition to having leading research in GLP-1 class peptide mimetics as small molecule agonists, Chugai Pharmaceutical also possesses a strong research foundation in KRAS and RAS class oral cyclic peptide inhibitors.
Searching Chugai's latest patents in the Synapse database revealed an application opened on November 9, 2023, titled: ' Cyclic compound having inhibitory effect selective for kras but not for hras and nras'. The application was filed on May 2, 2023, and was made public just six months later. The patent document is very extensive, containing synthesis examples of more than 3,000 cyclic peptides and detailing the Kd values for KRAS, NRAS, HRAS, as well as the inhibitory IC50 values on NCI-1441 cells.
The Kd value is calculated using Biacore, and the ratio of the Kd values of the other two subtypes to that of KRAS is denoted with letters. Ratios of Kd values greater than or equal to 0.1 and less than 3 are designated as C, ratios of 3 or higher and less than 10 are designated as B, ratios of 10 or higher and less than 20 are designated as A, and ratios of 20 or higher are designated as AA.
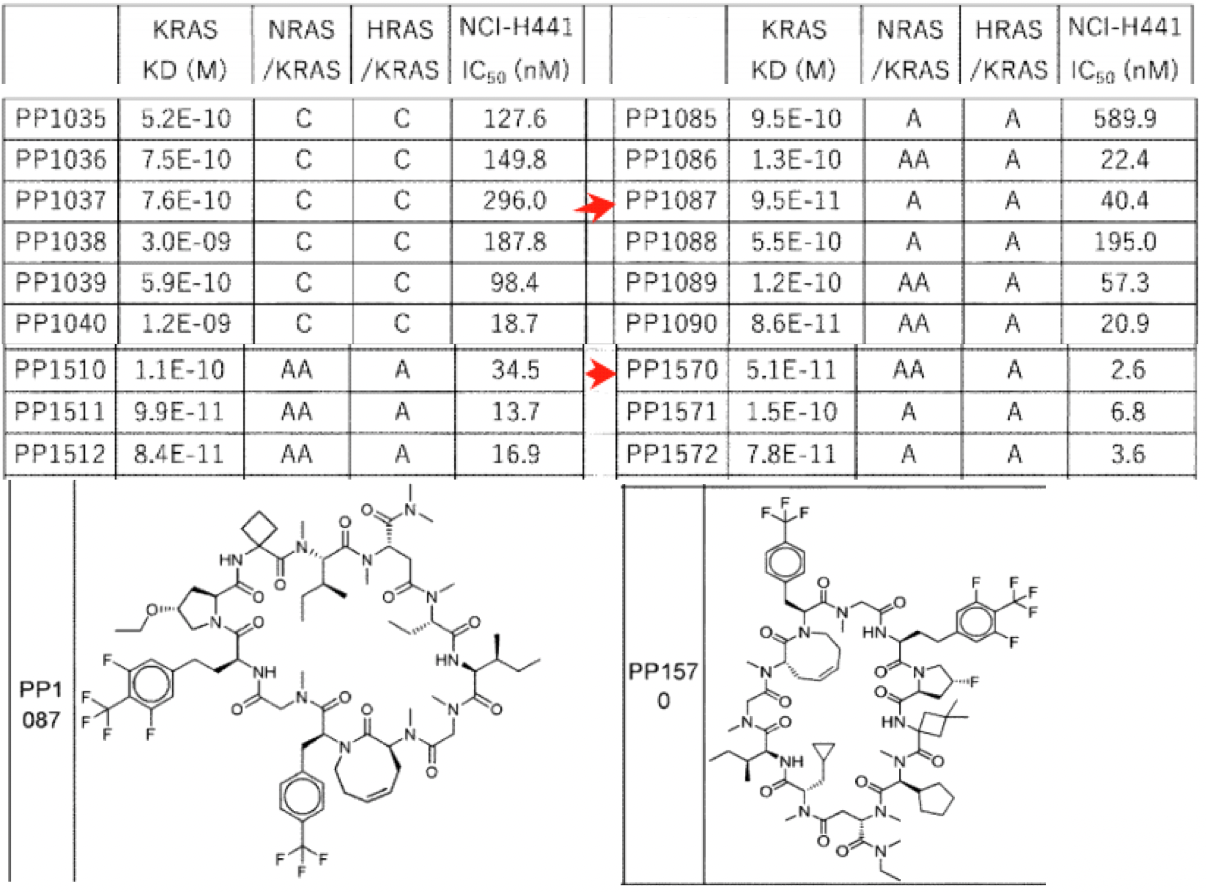
The figure above lists two implementation examples, PP1087 and 1570, both of which are 12mer peptides with KRAS Kd values in the range of 0.05-0.09 nM, indicating a high affinity. Their selectivity is over 10-fold, although no compounds with over 20-fold selectivity have been observed yet. The cyclic peptides in this patent are notably different from Chugai Pharmaceutical's clinical cyclic peptide LUNA18, though both are 12mers. Additionally, the two exemplified implementations each have only three hydrogen bond donors (NH), and the large ring conformation exhibits flexibility, which can enhance gastrointestinal absorption of the drug to meet the bioavailability requirements for oral medications.
In another patent by Chugai Pharmaceutical, WO2022234853, they disclosed their discovery of cyclic peptides and oligopeptide compounds that selectively inhibit KRAS. These peptidic compounds have been found to interact with specific amino acid residues of KRAS. The lead compound in this patent is PP-1501, with its selectivity determined through Kd values showing over 10-fold selectivity against HRAS and NRAS, and an IC50 value of 1.5 nM in inhibiting NCI-1441 cells. This is also a 12mer, containing three hydrogen bond donors and nine fluorine atoms within its molecule. This further illustrates that reducing the number of hydrogen bond donors/acceptors usually can improve the oral bioavailability of small molecules. Potentially reducing it further to below three might affect the activity and selectivity of this series of cyclic peptides, which is why Chugai Pharmaceutical consistently maintains three hydrogen bond donors in their cyclic peptides.

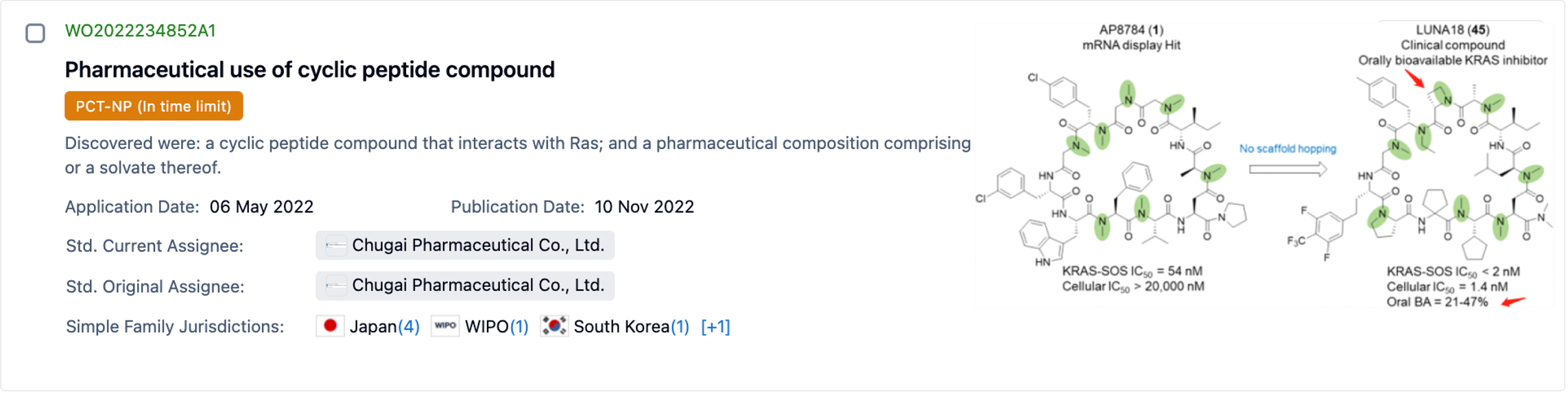
In another pharmaceutical patent, WO2022234852, the inclusion of their clinical cyclic peptide molecule LUNA-18 is described. Aside from AsPC-1, LUNA-18 exhibited significant cytotoxic activity (IC50=0.17-2.9nM) against cell lines with KRAS gene mutations, such as LS180 (colorectal cancer, KRASG12D), GSU (gastric cancer, KRASG12D), NCI-H441 (non-small cell lung cancer, KRASG12V), NCI-H2122 (non-small cell lung cancer, KRASG12C), and MiaPaCa-2 (pancreatic cancer, KRASG12C).
The in vivo efficacy of LUNA-18 was evaluated in NCI-H441 or MiaPaCa-2 xenograft models in mice. The compound LUNA-18 was administered orally once daily for a continuous period of 14 days, during which mouse body weight and tumor volume were measured. At a dose of 10 mg/kg, tumor regression was observed, with no significant weight loss noted.
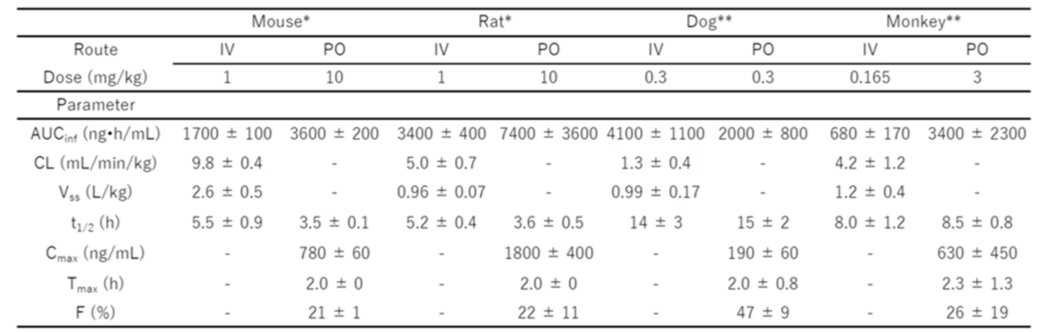
PK studies of luna18 were conducted in mice, rats, monkeys, and dogs. Following oral administration, the bioavailability of the compound luna18 in these four species ranged from 21% to 47%. On the 24th of last month, the Journal of the American Chemical Society (JACS) published the latest paper on the modification of a cyclic peptide inhibitor by Chinese and international pharmaceutical companies, in which the oral bioavailability of LUNA18 in various species was disclosed. In mice, a 10 mg/kg oral dose resulted in an exposure of 3600 ng*h/mL and a C_max of 780 ng/mL, indicating very good activity and an expectation to cover the concentration required for pharmacological efficacy. Rats showed slightly better performance compared to mice, with the C_max and AUC of IV and PO administration showing a doubling in growth, yet the bioavailability was only around 20%. Both dogs and monkeys exhibited longer half-lives, reaching 14 hours and 8 hours respectively, suggesting that the human oral half-life could also be over 10 hours and supporting once daily (QD) dosing in the clinic. Overall, mice, rats, dogs, and monkeys all showed low clearance, and improved absorption might further increase bioavailability (F). These results suggest that luna18, as an orally-administered cyclic peptide, holds therapeutic potential for treating various cancers carrying KRAS mutations. Currently, luna18 is in Phase 1 clinical trials in Japan and the USA.
Due to the KRAS signaling pathway functioning through intracellular PPIs, usually involving large binding surfaces, medicinal chemists are also pursuing macrocyclic formats, including nucleic acids, peptides, antibodies, or non-immunoglobulin proteins.
Cyclic peptides have attracted the attention of numerous companies. They are generally larger (500-3000 Da) than conventional small molecules, better suited to exploit the flat and hydrophobic interfaces characteristic of PPIs, and can exhibit antibody-like binding affinity and specificity with just a small fraction of their molecular weight. This is the theoretical basis for this class of macrocyclic peptide inhibitors to show high selectivity and fewer off-target side effects. Cyclic peptides also retain many desirable small molecule properties, such as low immunogenicity and resistance to proteolytic degradation.
Chugai Pharmaceutical is undeniably a pioneer in the development of oral RAS and KRAS peptide inhibitors. After transferring a GLP-1 oral small molecule agonist (peptidomimetic) to Eli Lilly, they continue to make innovative strides in KRAS cyclic peptides using their peptide development platform, where each patent comprises several thousand embodiments, and each embodiment requires completion of over four biological tests. This craftsmanship is highly admirable and has yielded significant rewards.