Otsuka Reveals Updates on Sibeprenlimab for Adult IgA Nephropathy Treatment
Otsuka Pharmaceutical Development & Commercialization, Inc. (OPDC) and Otsuka Pharmaceutical Co., Ltd. (Otsuka) have announced their intention to file a Biologics License Application (BLA) in the United States for sibeprenlimab, which is an investigational treatment for immunoglobulin A nephropathy (IgA nephropathy) in adults, aiming for submission in the first half of 2025. This announcement comes after a productive meeting with the U.S. Food & Drug Administration regarding the encouraging interim analysis findings from the Phase 3 VISIONARY study (NCT05248646).
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
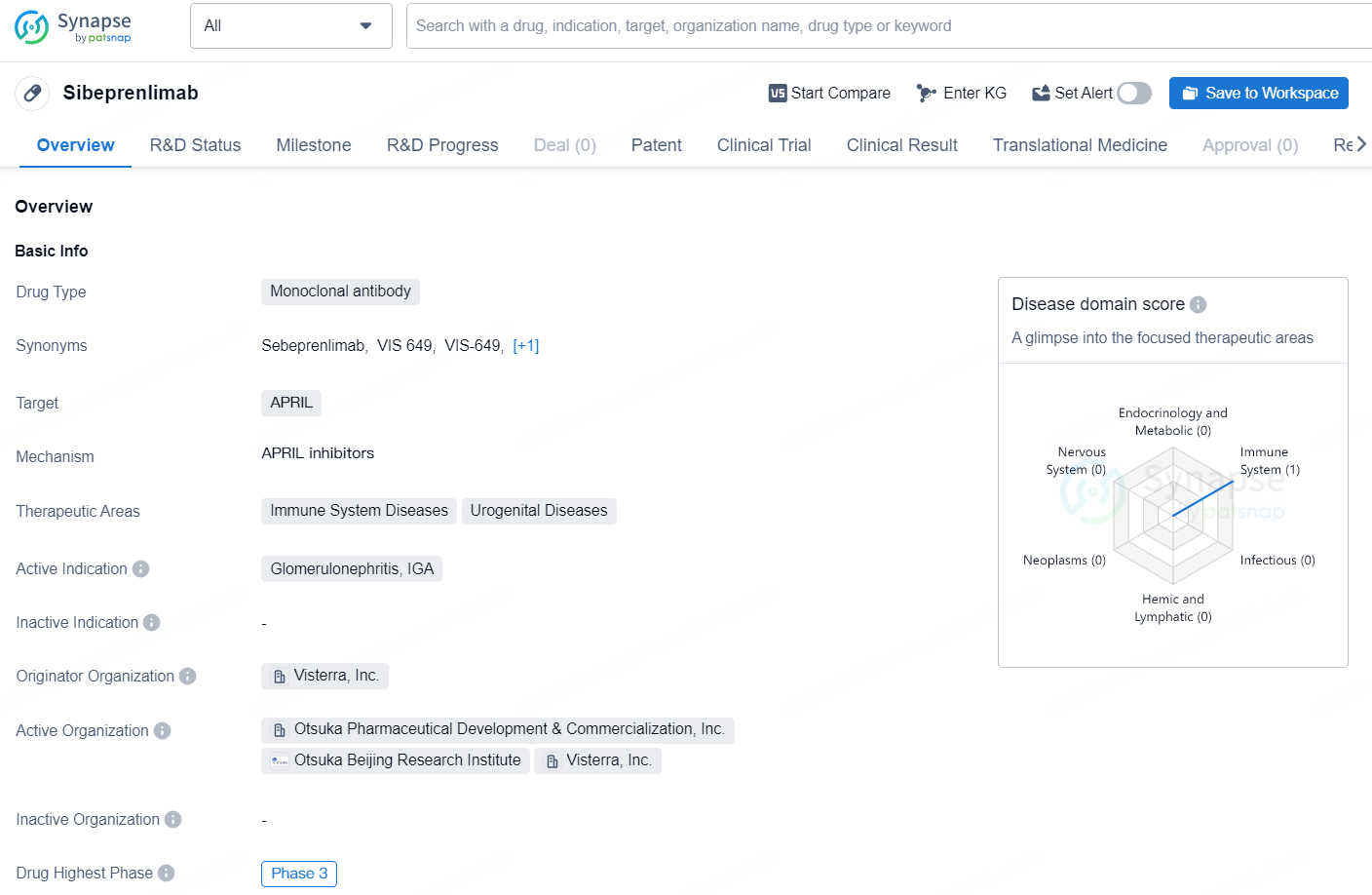
Sibeprenlimab is an experimental monoclonal antibody targeting APRIL (A PRoliferation-Inducing Ligand), designed to inhibit a crucial early step in the immune pathogenic process associated with IgA nephropathy. This action reduces the production of aberrant IgA1 (Gd-IgA1) and the subsequent formation of immune complexes. IgA nephropathy is a chronic, autoimmune kidney disorder that often progresses to end-stage kidney disease (ESKD) in many patients over their lifetime. Otsuka previously received Breakthrough Therapy designation for sibeprenlimab after promising outcomes from the Phase 2 ENVISION trial.
“We are grateful for the chance to share the encouraging interim findings from the Phase 3 trial of sibeprenlimab with the FDA, and we intend to integrate the Agency's insights as we move forward with our BLA submission,” said John Kraus, M.D., Ph.D., executive vice president and chief medical officer at Otsuka Pharmaceutical Development & Commercialization, Inc. “Otsuka remains dedicated to the nephrology field and is eager to provide this innovative targeted therapy to individuals suffering from IgA nephropathy. We extend our thanks to the patients, their families, and healthcare providers who play such a vital role in this journey.”
In October, Otsuka revealed positive interim outcomes from its Phase 3 trial of sibeprenlimab. The ongoing Phase 3 study is being conducted in a blinded fashion, assessing changes in kidney function over a 24-month period via estimated glomerular filtration rate (eGFR), with completion anticipated in early 2026. Additional prespecified and exploratory analyses of the results will be performed to evaluate the complete therapeutic potential of sibeprenlimab in treating IgA nephropathy and will be shared subsequently.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
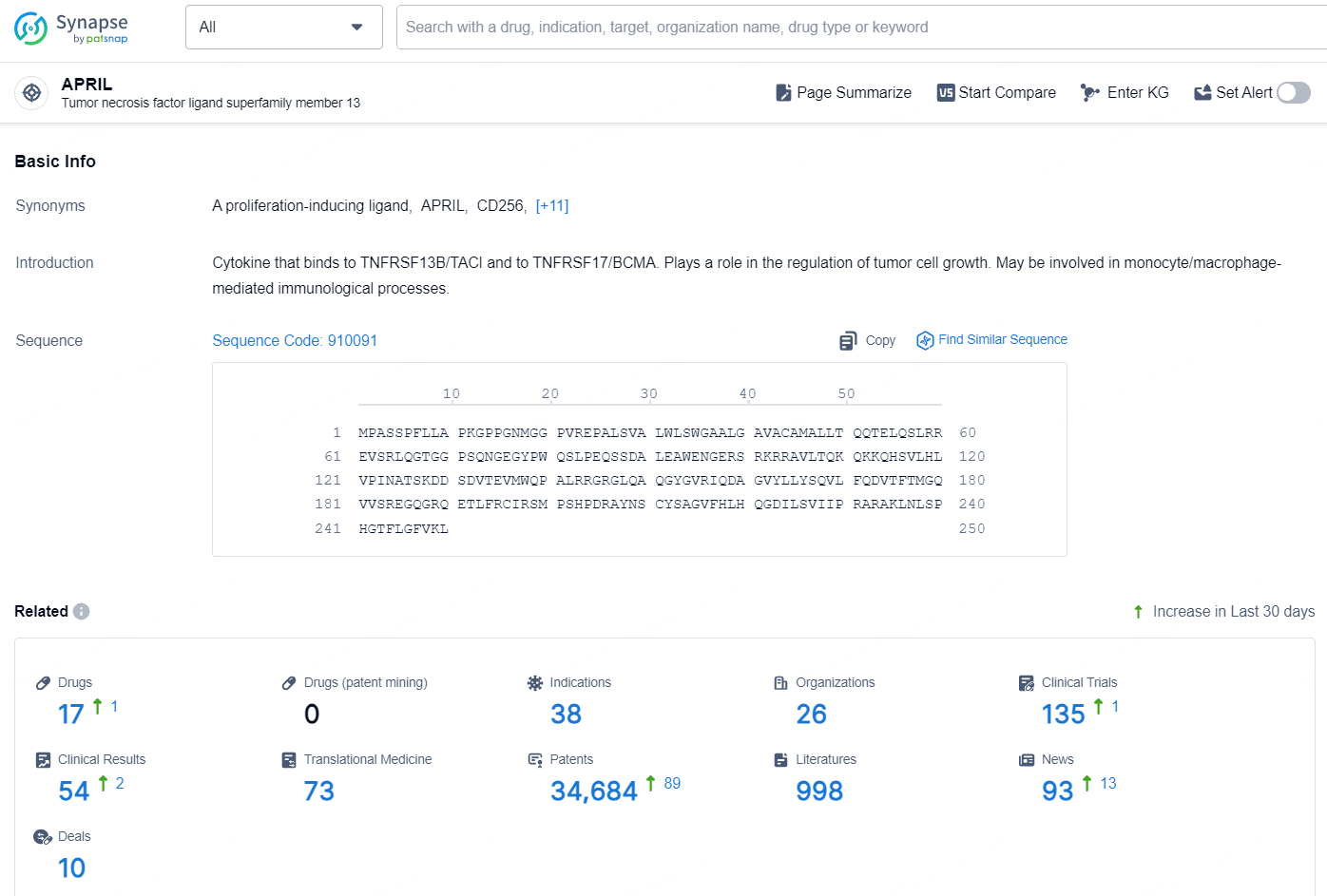
According to the data provided by the Synapse Database, As of November 20, 2024, there are 17 investigational drugs for the APRIL target, including 38 indications, 26 R&D institutions involved, with related clinical trials reaching 135, and as many as 34684 patents.
Sibeprenlimab is a monoclonal antibody drug developed by Visterra, Inc. that targets APRIL and is intended for the treatment of immune system diseases and urogenital diseases. The drug's active indication is for glomerulonephritis, specifically IgA glomerulonephritis. Sibeprenlimab has reached the highest phase of clinical development, which is Phase 3, both globally and in China.