Overview of Biogen’s Pipeline|R&D Progress
Biogen, Inc. is a biotechnology company based in Cambridge, Massachusetts, USA, specializing in the development of neurological diseases, autoimmune diseases, and cancer drugs. One of the world's first biotechnology companies founded in 1978 by Charles Weissmann, Heinz Schaller, Kenneth Murray and Nobel laureates Walter Gilbert and Phillip Sharp. In November 2017, Biogen Biotechnology (Shanghai) Co., Ltd. was registered in China. The company's core growth areas include multiple sclerosis (MS) and neuroimmunology; Alzheimer's disease (AD) and dementia; neuromuscular diseases, including spinal muscular atrophy (SMA) and amyotrophic lateral sclerosis (ALS); Movement disorders, including Parkinson's; and ophthalmology. The company is also focused on discovering, developing, and delivering innovative therapies globally in emerging growth areas such as immunology, neurocognitive disorders, acute neurology and pain.
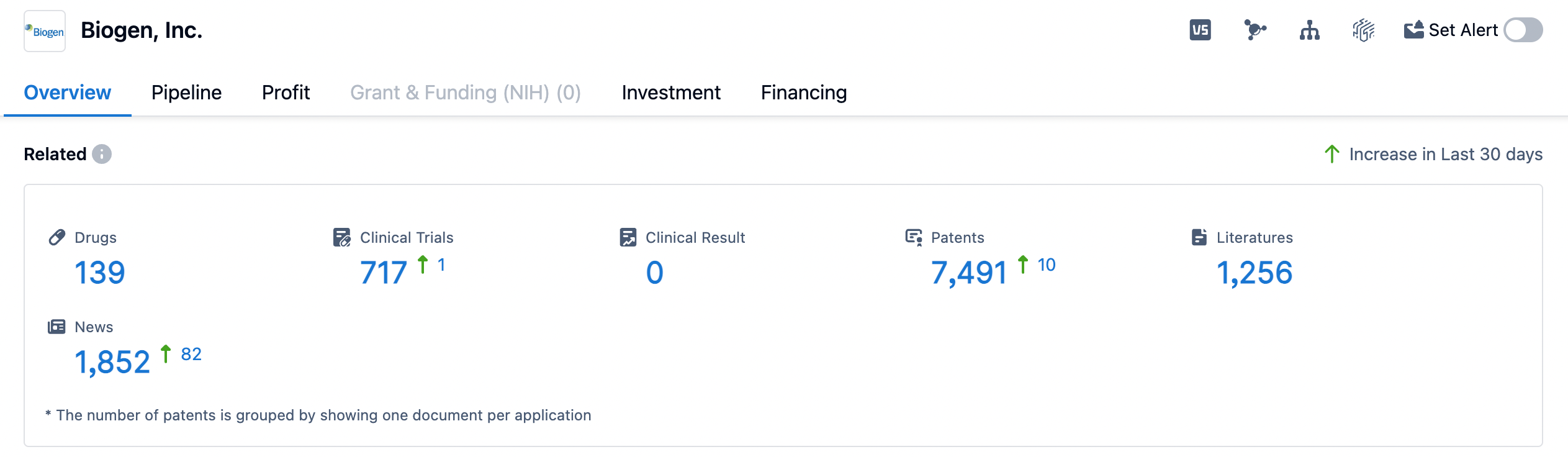
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of Biogen.
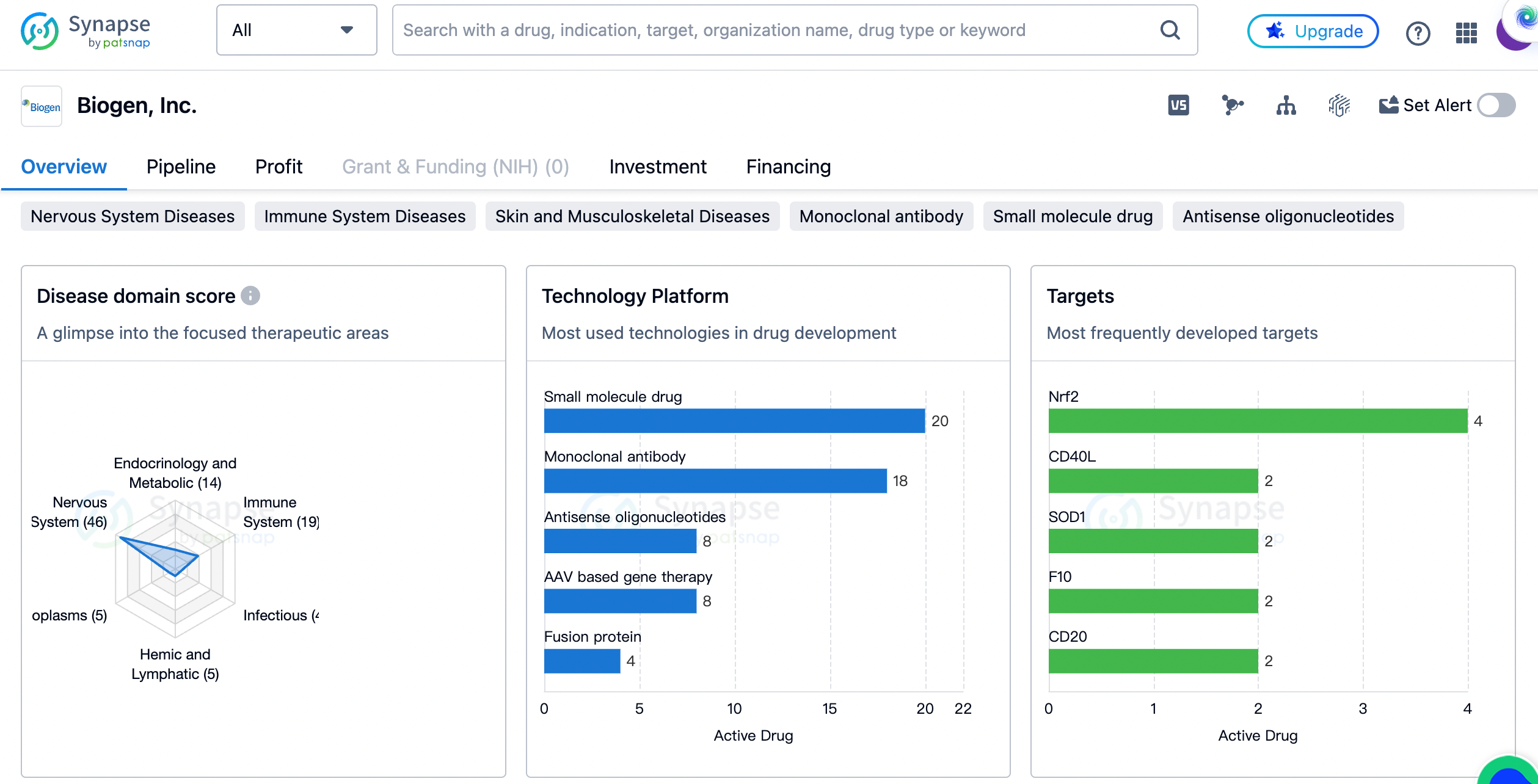
The distribution of therapeutic areas
Biogen has developed drugs for a wide range of therapeutic areas. The largest number of drugs developed by the company are for Nervous System Diseases, with a count of 46. This indicates that Biogen has a strong focus on developing treatments for neurological conditions. The second largest therapeutic area is Immune System Diseases, with 19 drugs developed. This suggests that Biogen is also actively involved in the development of treatments for immune-related disorders. Other therapeutic areas that Biogen has developed drugs for include Endocrinology and Metabolic Disease, Skin and Musculoskeletal Diseases, and Congenital Disorders, among others.
The most frequently developed targets by biogen
The target Nrf2 has the highest drug count of 4, indicating that Biogen has invested significant resources in developing drugs that target this particular protein. Other frequently developed targets include CD40L, SOD1, F10, CD20, Nav1.7, GABAA receptor, ACVR2B, APP, SMN2, and IFNAR, each with a drug count of 2. This suggests that Biogen has a diverse portfolio of drug targets, ranging from proteins involved in immune responses to those associated with neurological disorders.
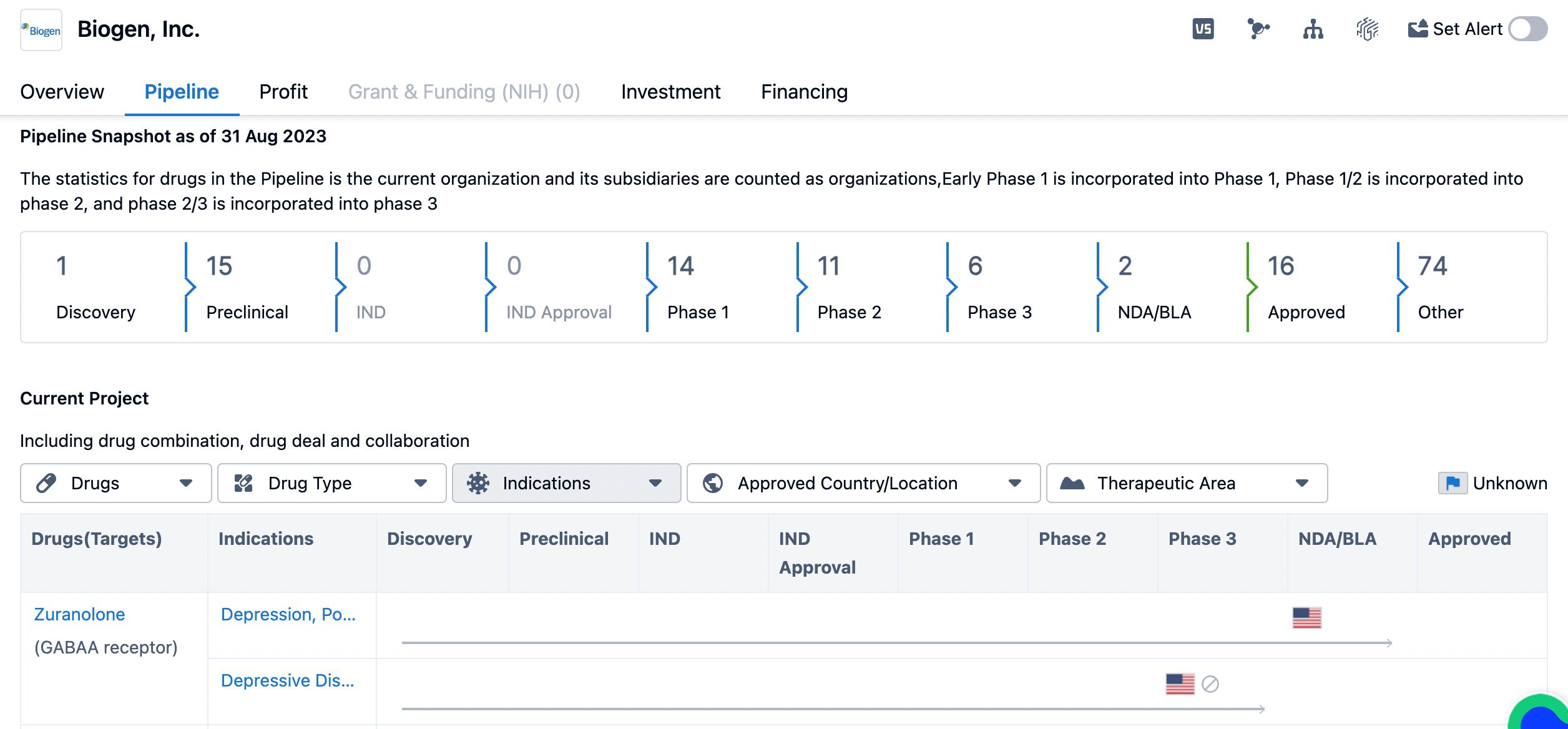
The overview of biogen's pipeline of drugs
The pipeline consists of drugs at various stages of development, starting from the Discovery phase to the Approved phase. As of the given date, Biogen has 1 drug in the Discovery phase, 15 drugs in the Preclinical phase, 14 drugs in Phase 1, 11 drugs in Phase 2, and 6 drugs in Phase 3. This indicates that Biogen has a robust pipeline of drugs in different stages of development, with a significant focus on early-stage research and clinical trials. The pipeline also includes 2 drugs in the NDA/BLA (New Drug Application/Biologics License Application) stage, indicating that Biogen has submitted these drugs for regulatory approval. Additionally, 16 drugs have already been approved, suggesting that Biogen has a track record of successfully bringing drugs to market. The pipeline also includes a significant number of drugs categorized as "Other," which could refer to drugs in various stages of development or those targeting different therapeutic areas.
In summary, Biogen, Inc. is a pharmaceutical company that was founded in 1978 and is based in Massachusetts, United States. The company has a strong focus on the development of drugs for Nervous System Diseases and Immune System Diseases, with a diverse portfolio of drugs targeting various proteins and pathways. Biogen has a robust pipeline of drugs at different stages of development, with a significant emphasis on early-stage research and clinical trials. The company has also successfully obtained regulatory approval for several drugs, indicating its ability to bring treatments to market. Overall, Biogen's focus on neurological and immune-related disorders, along with its diverse pipeline, positions it as a key player in the pharmaceutical industry.