Pamrevlumab Trial Results in Advanced Pancreatic Cancer: FibroGen's Update
FibroGen, Inc. released preliminary findings from two advanced-phase studies assessing the effectiveness and safety of pamrevlumab in individuals with pancreatic cancer, along with a company update.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The experimental arm of the Precision Promise Phase 2/3 adaptive platform trial by PanCAN assessed the combination of pamrevlumab with gemcitabine + nab-paclitaxel against gemcitabine + nab-paclitaxel alone in first and second line metastatic pancreatic ductal adenocarcinoma patients. The pamrevlumab cohort did not achieve the primary endpoint of overall survival as specified by the predetermined Bayesian statistical analysis in the trial protocol.
In the Phase 3 LAPIS trial, the efficacy of pamrevlumab in combination with gemcitabine + nab-paclitaxel or FOLFIRINOX was compared to a placebo combined with gemcitabine + nab-paclitaxel or FOLFIRINOX for treating locally advanced, unresectable pancreatic cancer. Similar to the other study, this trial also did not meet its primary endpoint of overall survival.
In response to these outcomes from the late-stage trials for pamrevlumab in pancreatic cancer, the Company intends to initiate an immediate and substantial cost reduction plan within the U.S. This includes the discontinuation of the pamrevlumab development program and the swift wind-down of pending obligations related to pamrevlumab. Consequently, the Company's U.S. workforce will be reduced by approximately 75%.
Preliminary safety analyses from both trials indicate that pamrevlumab, when combined with gemcitabine + nab-paclitaxel or FOLFIRINOX, was generally well tolerated by pancreatic cancer patients, showing an acceptable safety profile. No significant differences in treatment-emergent adverse events were observed between the different treatment groups.
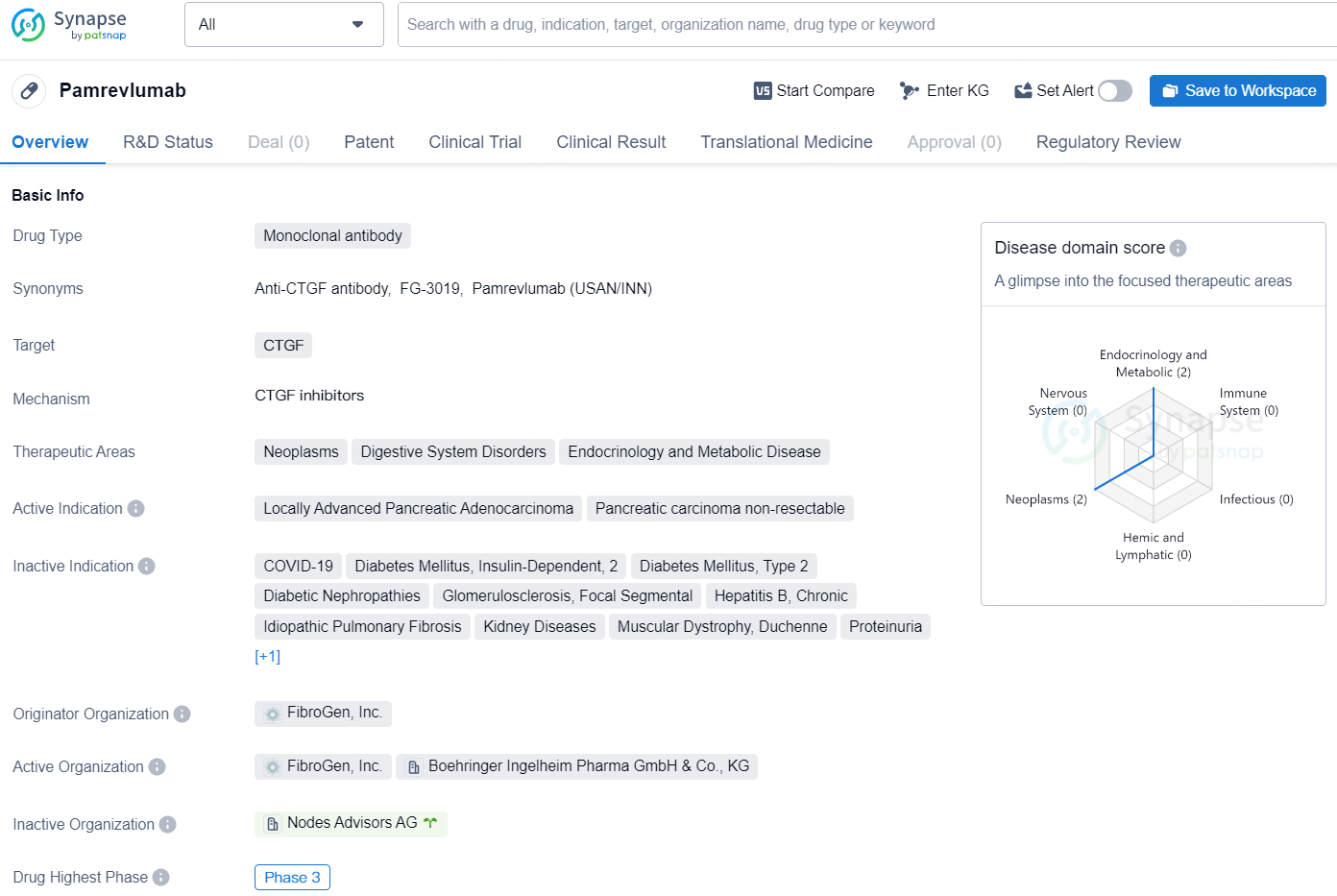
Pamrevlumab, a potential first-in-class anti-CTGF fully human monoclonal antibody developed by FibroGen, aims to inhibit the activity of connective tissue growth factor (CTGF). It is under clinical development for treating metastatic pancreatic cancer as well as locally advanced unresectable pancreatic cancer.
The U.S. Food and Drug Administration has granted Orphan Drug Designation for pamrevlumab in treating patients with pancreatic ductal adenocarcinoma and Fast Track designation for treating patients with LAPC. It remains an investigational drug and has not received marketing approval from any regulatory authority.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
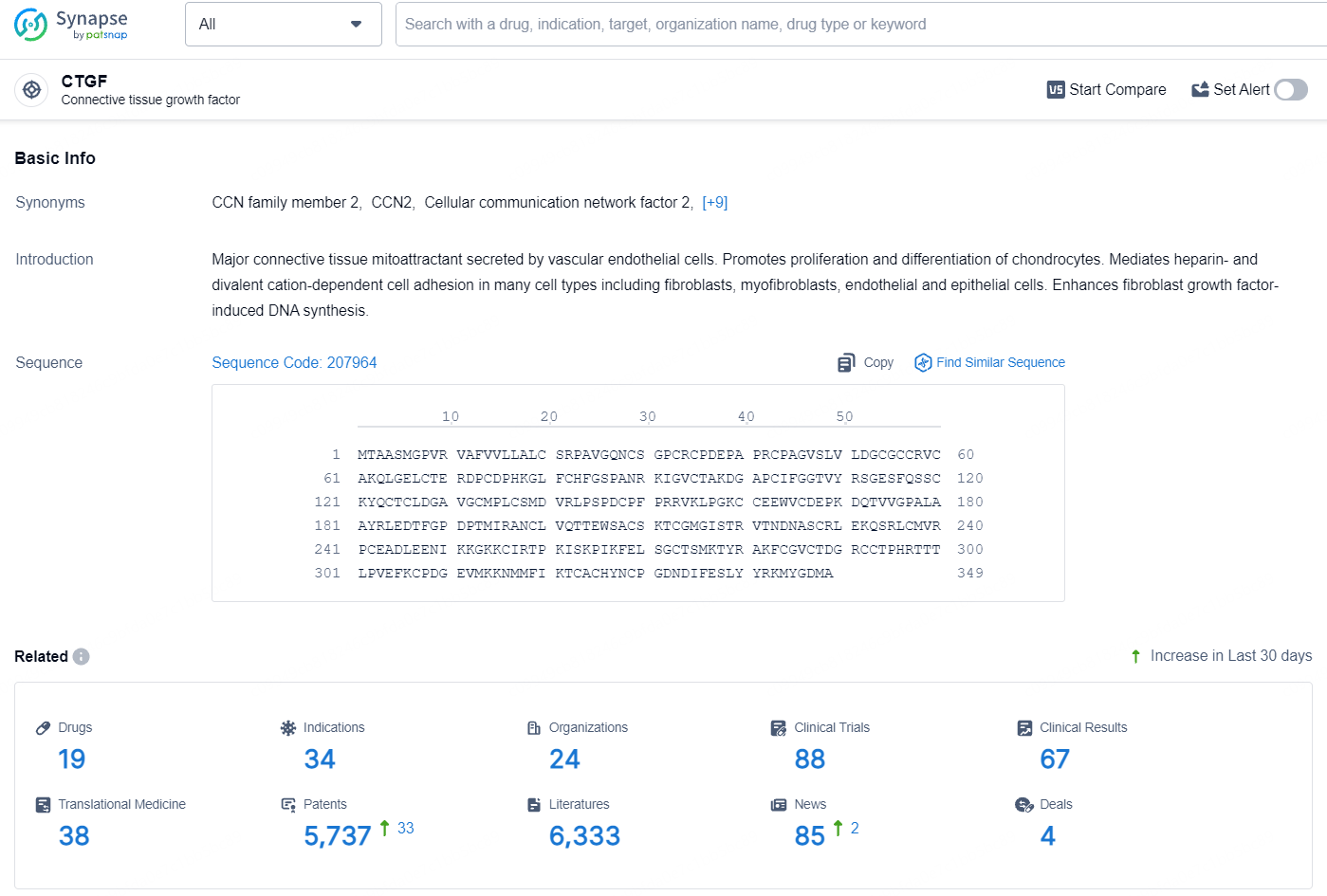
According to the data provided by the Synapse Database, As of August 5, 2024, there are 19 investigational drugs for the CTGF target, including 34 indications, 24 R&D institutions involved, with related clinical trials reaching 88, and as many as 5737 patents.
The development of Pamrevlumab represents an important advancement in the field of biomedicine, particularly for patients with locally advanced pancreatic adenocarcinoma and non-resectable pancreatic carcinoma. The drug's specific targeting of CTGF and its therapeutic potential in various disease areas provide hope for improved treatment options for patients with these conditions.