Patent Drafting Pitfalls: Alnylam's Custom Definition Sinks COVID-19 Vaccine Lawsuit
On June 4, 2025, the United States Court of Appeals for the Federal Circuit (CAFC) issued a precedential decision in a patent infringement case involving mRNA-based COVID-19 vaccines, addressing whether the patentee’s “custom” definition of a term constituted infringement.
Ultimately, the CAFC upheld the district court’s ruling that the defendant, Moderna, did not infringe. The case stemmed from the fact that the plaintiff, Alnylam Pharmaceuticals, had employed a self-defined interpretation of the term “branched alkyl” in two asserted patents—U.S. Patent Nos. 11,246,933 and 11,382,979.
The district court held that:
“Unless otherwise specified, the term ‘branched alkyl’... refers to an alkyl group where (1) a carbon atom in the group is bonded to at least three other carbon atoms; and (2) that carbon atom is not a ring atom of a cyclic group.”
Under this interpretation, Moderna’s product did not meet the requirement of having a “branched alkyl” group in which a carbon atom is connected to at least three other carbon atoms. As a result, the court found that there was no infringement.
Upon review, the CAFC agreed that Alnylam had, under the “unless otherwise specified” condition, adopted a lexicographic definition of “branched alkyl” requiring the carbon atom to be bonded to at least three other carbon atoms. Since Alnylam did not provide an alternative definition within the claims at issue, the CAFC affirmed the district court’s judgment of non-infringement.
The case began in March 2022 when Alnylam sued Moderna in the U.S. District Court, alleging that Moderna’s COVID-19 vaccine, SPIKEVAX, infringed claim 18 and other claims of the ’933 patent relating to the SM-102 lipid.
After the issuance of the ’979 patent, a continuation of the ’933 patent, in July 2022, Alnylam filed a second lawsuit alleging infringement of claim 1 and others.
Both parties subsequently submitted competing claim constructions for the terms “branched alkyl” and “branched C10–C20 alkyl.”
Alnylam proposed the ordinary meaning of “branched alkyl” as “a non-linear saturated hydrocarbon moiety” and argued that “branched C10–C20 alkyl” should encompass alkyl groups containing 10 to 20 carbon atoms.
Moderna, on the other hand, advocated for the definition found in column 12 of the patent specification:
“An alkyl group in which (1) a carbon atom is bonded to at least three other carbon atoms, and (2) that carbon atom is not a ring atom of a cyclic group.”
In August 2023, the district court sided with Moderna, ruling that the relevant portions of the ’933 patent constituted a “clear and unambiguous lexicographic definition.” The court explained that deviating from such a definition would require a clear and unmistakable indication to the contrary—something Alnylam failed to demonstrate either in the claims or in the written description.
Alnylam subsequently appealed to the CAFC.
Alnylam argued that the district court erred in concluding that it had acted as a lexicographer, asserting that intrinsic evidence indicated no intent to limit “branched alkyl” to scenarios where a carbon atom is connected to three others. It further argued that the district court’s construction excluded disclosed embodiments.
The CAFC rejected these arguments, finding no basis to conclude that the term “branched alkyl” as used in the claims encompassed α-secondary carbon atoms—an interpretation that would contradict the specification’s express definition.
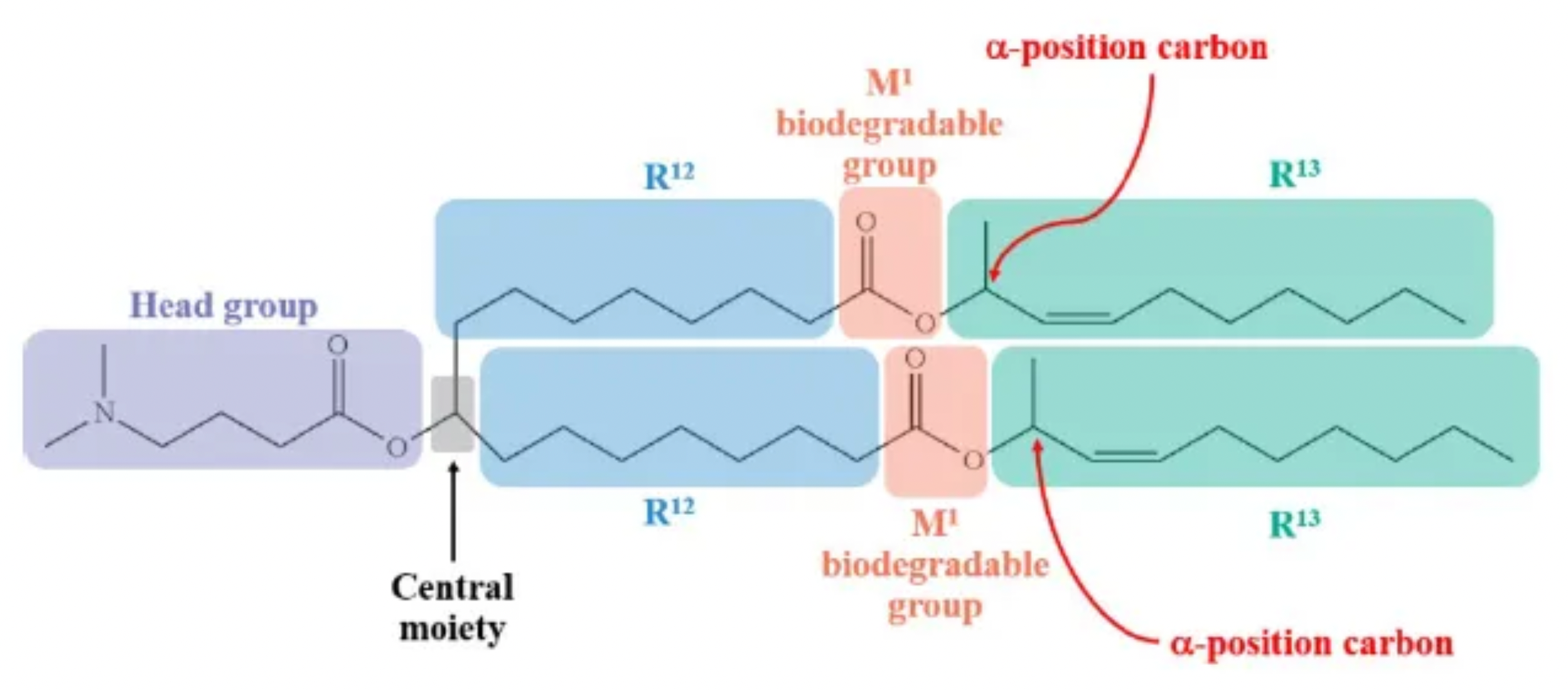
Alnylam contended that the asserted claims “cover” or are compatible with branched alkyl groups containing either a secondary carbon at the α-position (i.e., bonded to two other carbon atoms) or a carbon bonded to three other carbon atoms. In support of this position, Alnylam pointed to unasserted claim 14 of the ’933 patent. Citing the general principle that an independent claim is typically broader in scope than its dependent claims, Alnylam argued that the independent claim should be interpreted to encompass α-position secondary carbon atoms bonded to only two other carbon atoms.
The CAFC rejected this argument, finding that dependent claim 14 narrows the scope of the independent claim, and thus Alnylam’s position was inconsistent with the established interpretive principle.
In the specification, Alnylam highlighted several embodiments that include a secondary carbon atom at the α-position. However, the CAFC noted that nearly all of these embodiments fall outside the scope of the asserted claims, and therefore, they could not serve to redefine the term “branched alkyl.”
Finally, Alnylam cited a passage from the definition section stating that “representative saturated branched alkyl groups include isopropyl, sec-butyl, isobutyl, tert-butyl, and isopentyl,” arguing that both isopropyl and sec-butyl contain a secondary carbon atom at the α-position. The CAFC, however, found this unpersuasive because those examples do not contain the 10–20 carbon atoms required by the claims and therefore lie outside the scope of the asserted claims.
Although the CAFC acknowledged that the prosecution history most nearly suggested that Alnylam may have considered “branched alkyl” to include secondary carbon atoms, it ultimately concluded that the prosecution history was insufficient to override the express definition provided in the specification.
This case serves as another recent example in the United States regarding the treatment of custom-defined terms in patents. It reinforces the principle that when patentees choose to define claim terms in a customized manner, they must do so with clear and unambiguous language that enables a person skilled in the art to understand the intended meaning—otherwise, they must bear the adverse consequences resulting from such definitions.
Discover Eureka LS: AI Agents Built for Biopharma Efficiency
Stop wasting time on biopharma busywork. Meet Eureka LS - your AI agent squad for drug discovery.
▶ See how 50+ research teams saved 300+ hours/month
From reducing screening time to simplifying Markush drafting, our AI Agents are ready to deliver immediate value. Explore Eureka LS today and unlock powerful capabilities that help you innovate with confidence.