The Radiopharmaceutical Revolution: Transforming Precision Diagnosis to Personalized Therapy
Radiopharmaceuticals, which combine radioactive isotopes with specific molecular carriers, are ushering in a new era of personalized medicine. These specialized drugs precisely target diseased tissues within the human body and release ionizing radiation for diagnostic or therapeutic purposes, significantly enhancing the accuracy and effectiveness of disease management. From traditional thyroid function tests to their current widespread application in cancer, cardiovascular diseases, and neurodegenerative disorders, the development of radiopharmaceuticals reflects how advancements in science and technology are driving innovation in medicine.
With the advent of cutting-edge technologies such as radioligand therapies (RDCs), radiopharmaceuticals are not only improving the accuracy of tumor diagnostics but also offering new treatment options for previously intractable diseases. Notably, innovative drugs such as 177Lu-PSMA-617 and 18F-PSMA-1007 are leading the way in precision medicine for malignancies like prostate cancer and renal cell carcinoma.
Mechanism of Action of Radiopharmaceuticals
Radiopharmaceuticals, also known as radioactive drugs or radionuclide formulations, are specialized agents that utilize radioactive isotopes bound to specific molecular carriers for medical diagnosis and treatment. These compounds can selectively target specific areas of the body—such as tumors or other diseased tissues—and emit ionizing radiation to fulfill their intended function.
Over the past decades, the application of radiopharmaceuticals has expanded from traditional thyroid diagnostics to the treatment and imaging of cancer, cardiovascular conditions, and neurodegenerative diseases. With technological progress, especially in radioligand therapy, radiopharmaceuticals can act more precisely on target cells while minimizing damage to surrounding healthy tissues.
The introduction of alpha-particle emitters has brought new hope to therapy, thanks to their higher cytotoxicity and shorter range, making them especially suitable for hard-to-treat tumors.
The mechanism by which radiopharmaceuticals function in cancer treatment relies on the ionizing radiation emitted by radioactive isotopes. This radiation damages the DNA of cancer cells, inhibiting their division and proliferation. Different isotopes emit various types of radiation, such as beta particles or alpha particles, each affecting cancer cells differently.
Beta particles, which have moderate penetration ability, can affect cancer cells several cell diameters away, creating a "crossfire effect" that extends the therapeutic range. In contrast, alpha particles possess extremely high energy density and short range, enabling them to inflict severe damage within a limited area—often requiring only a few alpha particles to pass through a cancer cell nucleus to achieve cytotoxicity.
One of the key advantages of radiopharmaceuticals lies in their high specificity and low systemic toxicity. By conjugating radioactive isotopes to targeting ligands—such as antibodies or specific peptides—the isotopes can accumulate selectively in tumor sites, substantially increasing the local radiation dose while minimizing exposure to healthy tissue. This strategy not only improves therapeutic efficacy but also reduces the incidence of side effects.
In addition, some radiopharmaceuticals can also serve diagnostic purposes, allowing physicians to monitor disease progression in real time during treatment—thus achieving a theranostic (therapy + diagnostic) approach.
Another benefit is their low likelihood of inducing drug resistance. Because their mechanism of action differs from that of conventional chemotherapeutics, cancer cells have a harder time developing resistance. Therefore, radiopharmaceuticals can be used as standalone therapies or in combination with other modalities, such as immunotherapy, to enhance overall treatment outcomes.
Radioligand therapy (RLT), also known as radioimmunotherapy or radionuclide-conjugated drug therapy, involves binding a radioactive isotope to a molecule that targets specific cancer cells. This approach uses the emitted particles from radioactive substances to damage cancer cell DNA, thereby halting their growth and division. RLT plays a pivotal role in precision medicine, offering highly selective treatment of tumors while minimizing collateral damage to healthy tissues.
The core of RLT lies in the use of radioligands—compounds composed of four key elements: a targeting moiety (e.g., antibody, peptide, or small molecule), a linker, a chelator, and a radioactive isotope. Once administered, these radioligands seek out and bind to overexpressed biomarkers on cancer cell surfaces. Upon binding, the isotope emits radiation that directly damages the cancer cell’s DNA, leading to cell death.
Additionally, the so-called “bystander effect”—in which ionization of nearby water molecules produces free radicals—can also contribute to the destruction of adjacent cancer cells.
Application of Radiopharmaceuticals in Cancer Treatment
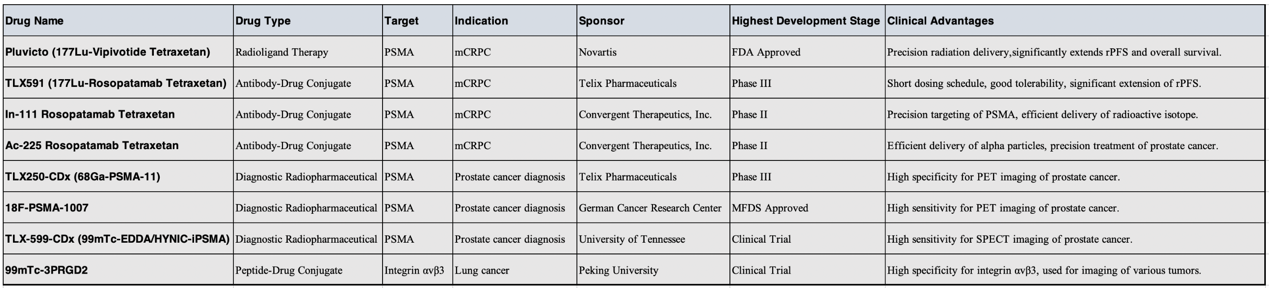
1)177Lu-PSMA-617
177Lu-PSMA-617 is an innovative targeted radioligand therapy specifically designed for the treatment of metastatic castration-resistant prostate cancer (mCRPC). This therapy achieves high selectivity and precision in targeting prostate cancer cells by combining Lutetium-177—a radioactive isotope that emits beta particles—with a small-molecule compound that specifically binds to prostate-specific membrane antigen (PSMA).
177Lu-PSMA-617 circulates in the bloodstream, identifying and binding to prostate cancer cells that overexpress PSMA. PSMA is highly expressed on the surface of prostate cancer cells and their metastases, while its expression in normal tissues is minimal. This allows the drug to effectively target cancer cells while sparing most healthy tissues. Once bound to PSMA on the surface of cancer cells, 177Lu-PSMA-617 is internalized. Inside the cell, Lutetium-177 emits beta particles that cause DNA damage, impairing the cancer cell's ability to replicate or inducing cell death directly. Although these beta particles are potent enough to destroy cancer cells, their limited penetration range—typically just a few millimeters—means that surrounding healthy tissues are largely unaffected. This reduces systemic side effects and enhances the safety profile of the treatment.
The VISION study demonstrated that 177Lu-PSMA-617 in combination with the best standard of care (SOC) provides significant benefits over SOC alone in terms of prolonging overall survival (OS) and radiographic progression-free survival (rPFS). Specifically, patients treated with 177Lu-PSMA-617 showed higher overall survival rates and longer progression-free periods, along with improved objective response rates and disease control rates.
Despite its high specificity and efficacy, 177Lu-PSMA-617 is not without side effects. For instance, salivary gland dysfunction may occur due to off-target uptake, as salivary glands also express PSMA. Therefore, during clinical application, physicians must closely monitor patients and take appropriate measures to manage and mitigate potential adverse effects.
2) 177Lu-Rosopatamab Tetraxetan
TLX591 (177Lu-Rosopatamab Tetraxetan) is another radiolabeled compound that targets prostate-specific membrane antigen (PSMA). It couples the radioactive isotope 177Lutetium with the monoclonal antibody rosopatamab tetraxetan. Rosopatamab tetraxetan specifically recognizes and binds to PSMA, which is overexpressed on the surface of prostate cancer cells. 177Lutetium serves as a beta particle emitter, delivering cytotoxic radiation that damages the DNA of cancer cells. Upon administration, rosopatamab tetraxetan seeks out and attaches to PSMA-positive prostate cancer cells, enabling 177Lutetium to emit beta radiation with sufficient energy to destroy nearby tumor cells while limiting damage to distant healthy tissue, thus preserving normal function.
Multiple completed Phase I and II clinical trials have shown that TLX591 has a favorable safety and efficacy profile, and have validated the effectiveness of optimized fractionated dosing regimens. The ProstACT SELECT study demonstrated that, in patients with progressive mCRPC who had already undergone treatment, the median radiographic progression-free survival (rPFS) following TLX591 therapy was 8.8 months, indicating significant clinical benefit. Moreover, TLX591 is currently being evaluated in a Phase III international, multicenter, randomized controlled trial named ProstACT GLOBAL—the first such study targeting PSMA-positive mCRPC patients—to further assess its efficacy and safety compared to standard-of-care therapies.
Grand Pharmaceutical has acquired the rights to develop and commercialize TLX591 in the Chinese market through a collaboration with Telix Pharmaceuticals. This strategic partnership not only facilitates the introduction of innovative therapies in China but also enhances the company’s global R&D and manufacturing capabilities, supporting the advancement of cutting-edge cancer diagnosis and treatment solutions. With TLX591's promising performance in the international market and its progressing rollout in China, it is expected to offer a new therapeutic option for prostate cancer patients in the coming years.
Application of Radiopharmaceuticals in Early Tumor Diagnosis
1) 18F-PSMA-1007
18F-PSMA-1007 is a positron emission tomography (PET) imaging agent specifically used for the diagnosis and staging of prostate cancer. This radiotracer targets prostate-specific membrane antigen (PSMA), which is highly expressed in prostate cancer cells, particularly in cases of recurrence or metastasis. By labeling a small-molecule PSMA inhibitor with the radioisotope fluorine-18 (18F), 18F-PSMA-1007 enables highly sensitive and specific detection of prostate cancer lesions.
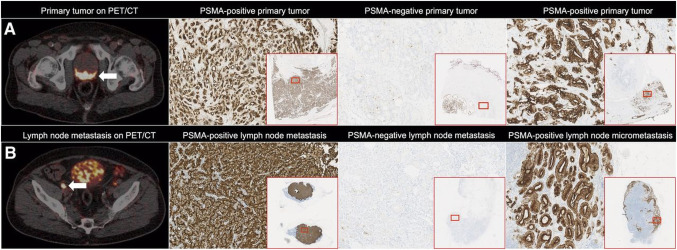
For patients who experience a rise in serum PSA levels after radical prostatectomy, recurrence is often suspected. However, traditional imaging techniques frequently fail to precisely localize recurrent lesions. 18F-PSMA-1007 PET/CT has demonstrated significant advantages in detecting small lesions in such patients due to its high sensitivity and specificity, enabling earlier detection of recurrence and timely adjustment of therapeutic strategies.
In patients with PSA levels in the “gray zone” (i.e., between 4 and 10 ng/mL), determining the presence of prostate cancer poses a diagnostic challenge. 18F-PSMA-1007 PET/CT, when combined with PSA-derived indicators such as PSA density (PSAD), can improve diagnostic accuracy in this group and reduce the number of unnecessary biopsies. This approach not only helps avoid overdiagnosis and overtreatment but also provides more precise therapeutic guidance for patients who do have prostate cancer.
By leveraging radiomics models based on 18F-PSMA-1007 PET/CT, researchers can extract numerous features from PET images and analyze them using machine learning algorithms to effectively distinguish prostate cancer from benign prostatic hyperplasia (BPH). This enhances diagnostic accuracy and contributes to more evidence-based clinical decision-making.
Studies also suggest that 18F-PSMA-1007 PET/CT can be used to predict the pathological grade of prostate cancer, which is critical for prognosis assessment. This imaging technique provides important insights into tumor biology, enabling the formulation of personalized treatment plans.
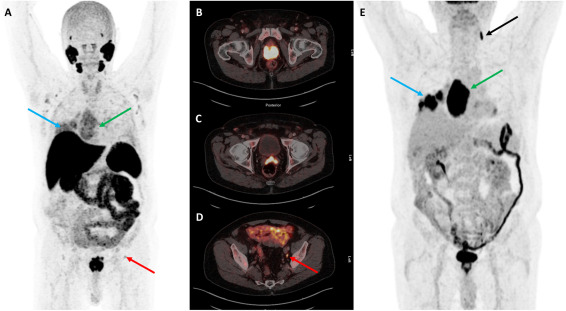
Compared to conventional 18F-FDG PET/CT, 18F-PSMA-1007 PET/CT offers higher sensitivity and specificity in detecting bone metastases from prostate cancer. This improves the detection of distant metastases, which is particularly important in managing advanced prostate cancer. Moreover, for suspected metastatic cases, the combined use of 18F-FDG and 18F-PSMA-1007 PET/CT can further enhance staging accuracy and guide clinical treatment decisions.
2) TLX250-CDx (89Zr-TLX250)
TLX250-CDx (89Zr-TLX250) is a radiopharmaceutical diagnostic conjugate (RDC) that targets carbonic anhydrase IX (CA9) and is specifically designed for the diagnosis of clear cell renal cell carcinoma (ccRCC).
Zirconium-89 (89Zr) is a positron-emitting isotope with a physical half-life of approximately 78.4 hours, making it well-suited for use with monoclonal antibodies due to its alignment with the biological distribution timeline of antibodies in the body. This allows 89Zr-labeled antibodies to achieve optimal tumor accumulation, thereby improving imaging sensitivity and specificity. The anti-CA9 antibody used in TLX250-CDx binds selectively to the CA9 protein, which is overexpressed on the surface of cancer cells. CA9 expression is minimal in normal tissues but significantly elevated in various cancers, especially ccRCC, making it an ideal diagnostic target.
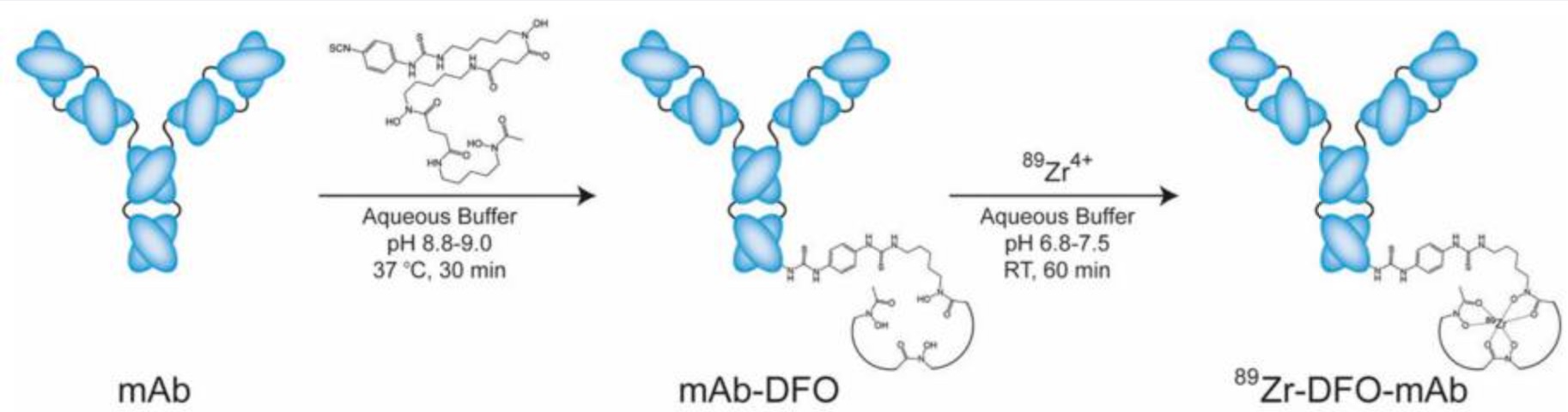
Once injected into the patient, TLX250-CDx circulates through the bloodstream and accumulates at tumor sites due to its high affinity for CA9. Using PET imaging, detailed in vivo images can then be generated, revealing the tumor’s location, size, and potential metastasis. This method not only improves diagnostic accuracy but also avoids the risks associated with invasive procedures such as biopsies.
In the pivotal Phase III ZIRCON clinical trial, TLX250-CDx demonstrated outstanding performance. For patients suspected of having ccRCC but not yet diagnosed, the agent achieved a sensitivity of 86% and specificity of 87% in diagnosing ccRCC, with a positive predictive value (PPV) of 93%. These results suggest that TLX250-CDx could become a vital non-invasive diagnostic tool, enabling more precise clinical decision-making. In addition to ccRCC, ongoing research is exploring the utility of TLX250-CDx in other CA9-expressing cancers, such as bladder cancer and urothelial carcinoma. These cross-indication studies could expand the market potential of TLX250-CDx and provide effective diagnostic solutions for a broader range of cancers.
ZIRCON is a global Phase III clinical trial designed to evaluate the efficacy and safety of TLX250-CDx in diagnosing ccRCC. The results have been encouraging: for patients with renal masses identified by CT or MRI but not definitively diagnosed as ccRCC, TLX250-CDx PET imaging showed 86% sensitivity and 87% specificity, with a PPV of 93%. These figures far exceed the U.S. FDA’s predefined thresholds (sensitivity and specificity ≥70%), indicating that TLX250-CDx has strong potential to become a new clinical diagnostic standard for ccRCC.
Beyond its diagnostic role in ccRCC, TLX250-CDx has shown promise in other cancer types. Studies are underway to assess its use in triple-negative breast cancer (TNBC), non-muscle-invasive bladder cancer (NMIBC), and urothelial carcinoma. If approved, TLX250-CDx would become the first commercially available imaging agent for ccRCC, addressing a significant unmet need. Given the often asymptomatic nature of early-stage kidney cancer, many patients are diagnosed at an advanced stage. Therefore, the early diagnostic capabilities provided by TLX250-CDx could substantially improve treatment outcomes and quality of life.
Conclusion
Radiopharmaceuticals offer tremendous potential in the diagnosis and treatment of cancer and other diseases due to their high selectivity and low systemic toxicity. They enhance diagnostic accuracy and enable earlier detection, while also providing safer and more effective therapeutic options for patients. For instance, 177Lu-PSMA-617 and TLX591 target prostate-specific membrane antigen (PSMA), bringing new hope to patients with metastatic castration-resistant prostate cancer (mCRPC); while 18F-PSMA-1007 and TLX250-CDx have shown remarkable success in the early diagnosis of prostate cancer and clear cell renal cell carcinoma (ccRCC), respectively. Furthermore, the use of these agents supports the development of theranostics—combining diagnosis and treatment—allowing clinicians to monitor disease progression in real-time and tailor treatment strategies to individual patient needs.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Reference
- 1.Chiliveri SC, Robertson AJ, Shen Y, Torchia DA, Bax A. Advances in NMR Spectroscopy of Weakly Aligned Biomolecular Systems. Chem Rev. 2022 May 25;122(10):9307-9330. doi: 10.1021/acs.chemrev.1c00730. Epub 2021 Nov 12. PMID: 34766756.
- 2.van der Gaag S, Bartelink IH, Vis AN, Burchell GL, Oprea-Lager DE, Hendrikse H. Pharmacological Optimization of PSMA-Based Radioligand Therapy. Biomedicines. 2022 Nov 23;10(12):3020. doi: 10.3390/biomedicines10123020. PMID: 36551776; PMCID: PMC9775864.
- 3.Jochumsen MR, Bouchelouche K. PSMA PET/CT for Primary Staging of Prostate Cancer - An Updated Overview. Semin Nucl Med. 2024 Jan;54(1):39-45. doi: 10.1053/j.semnuclmed.2023.07.001. Epub 2023 Jul 22. PMID: 37487824.
- 4.Yoon JK, Park BN, Ryu EK, An YS, Lee SJ. Current Perspectives on 89Zr-PET Imaging. Int J Mol Sci. 2020 Jun 17;21(12):4309. doi: 10.3390/ijms21124309. PMID: 32560337; PMCID: PMC7352467.