Patent Research and Operational Guide for Daiichi Sankyo's ADC Drug DS-8201(3)
Based on the characteristics of ADC drugs, we have summarized the process for conducting patent research on ADC drugs.You can start by reading the following article to get background information.
Patent Research and Operational Guide for Daiichi Sankyo's ADC Drug DS-8201(1)
Patent Research and Operational Guide for Daiichi Sankyo's ADC Drug DS-8201(2)
Finding Original Patents for Each Component Through Toxin Structure & Biological Sequence Searches
Toxin DX8951
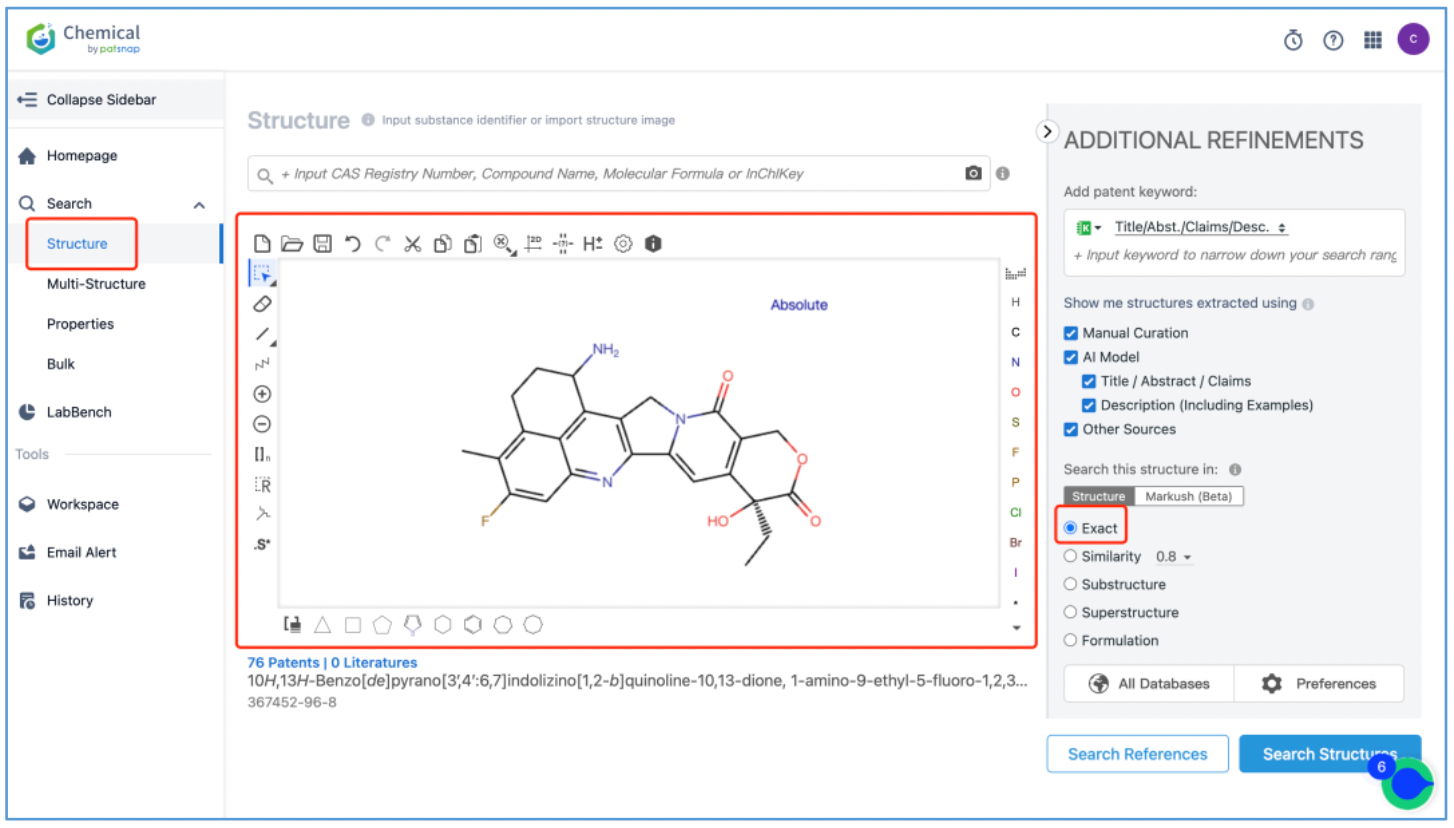
The DX8951 toxin is an analog of camptothecin, featuring a lactone ring that readily hydrolyzes and opens within the body. However, hydrolysis is impeded in the acidic environment of tumors. When conjugated with an anti-tumor antibody, its tendency for easy hydrolysis results in low toxicity and selective targeting of tumor cells, sparing normal tissues. To analyze the original patents for toxin DX8951, log in to Patsnap Chemical and draw its structure in the structure editor. Use the additional refinements on the right to edit the search criteria. For a targeted search, it is recommended to conduct an "exact search".
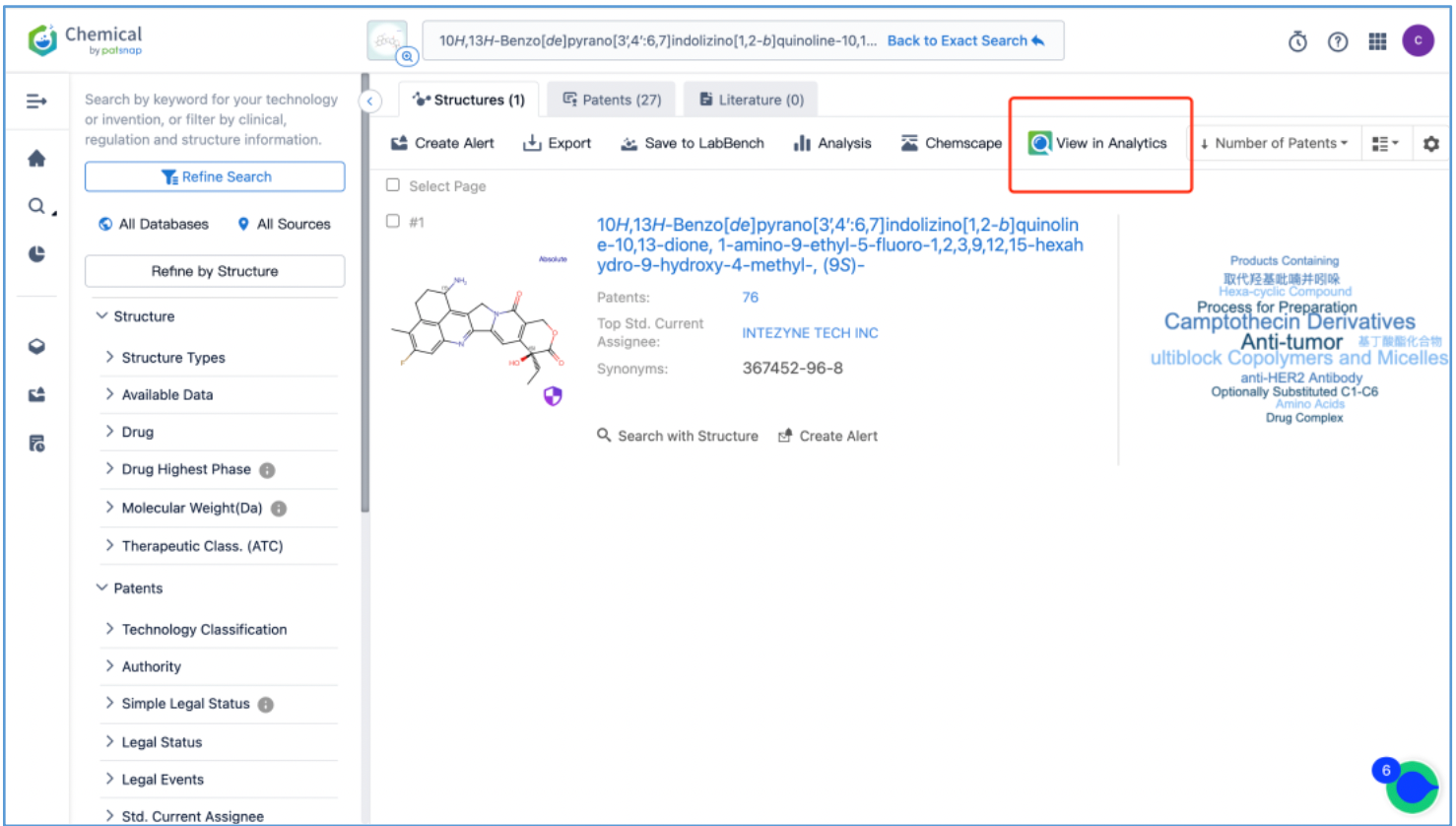
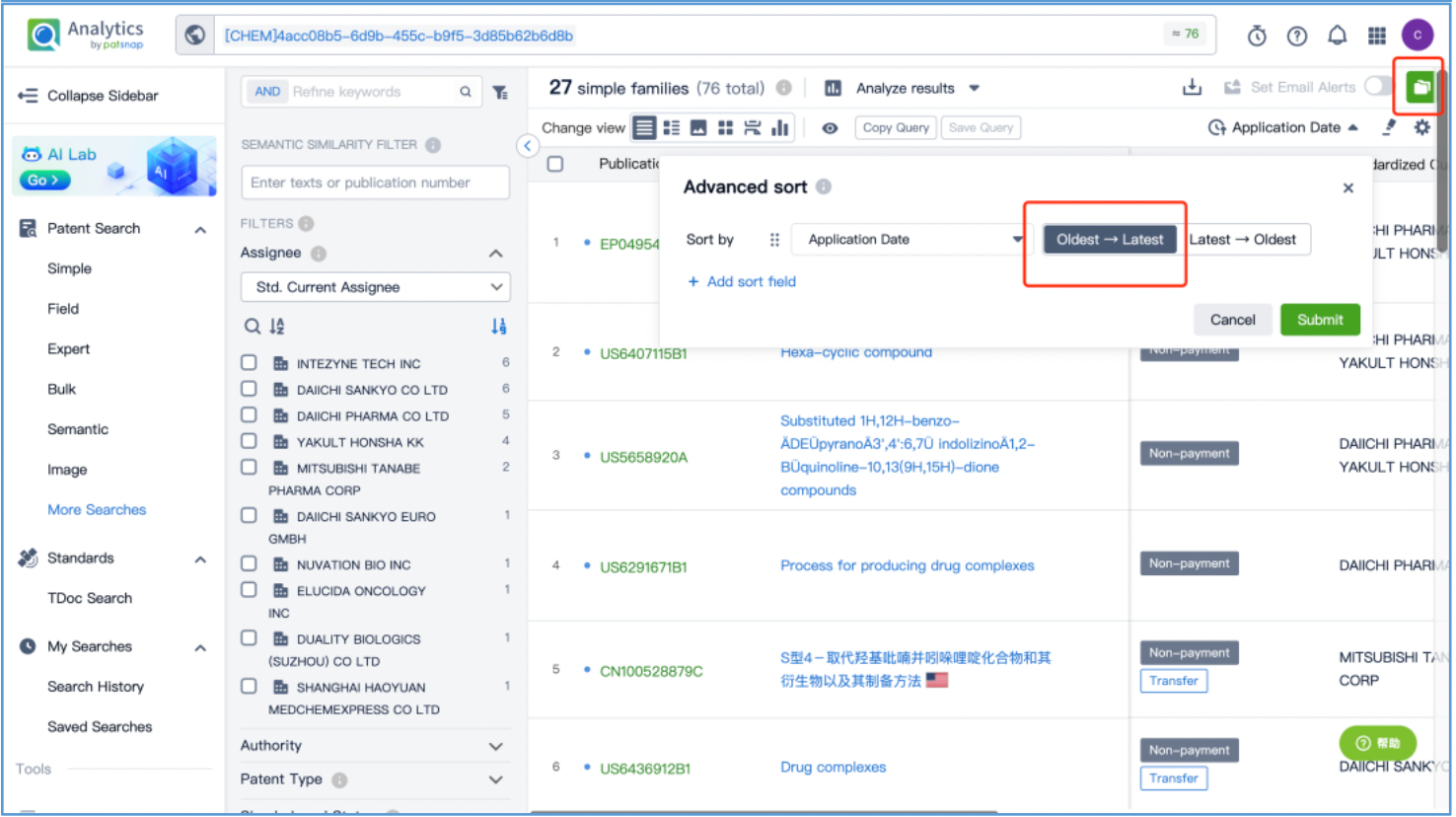
From the results, select the corresponding structure and click “View in Analytics” to launch the 76 related patents in Patsnap Analytics. Since the goal of this search is to find the original patents for DX8951, sort the results by application date from oldest to latest to quickly locate the original patent applications. By reviewing the claims and descriptions of the top patents, the original Markush US patent is identified as US6407115B1 and its original specific compound US patent as US5658920A. Save these patents to Workspace.
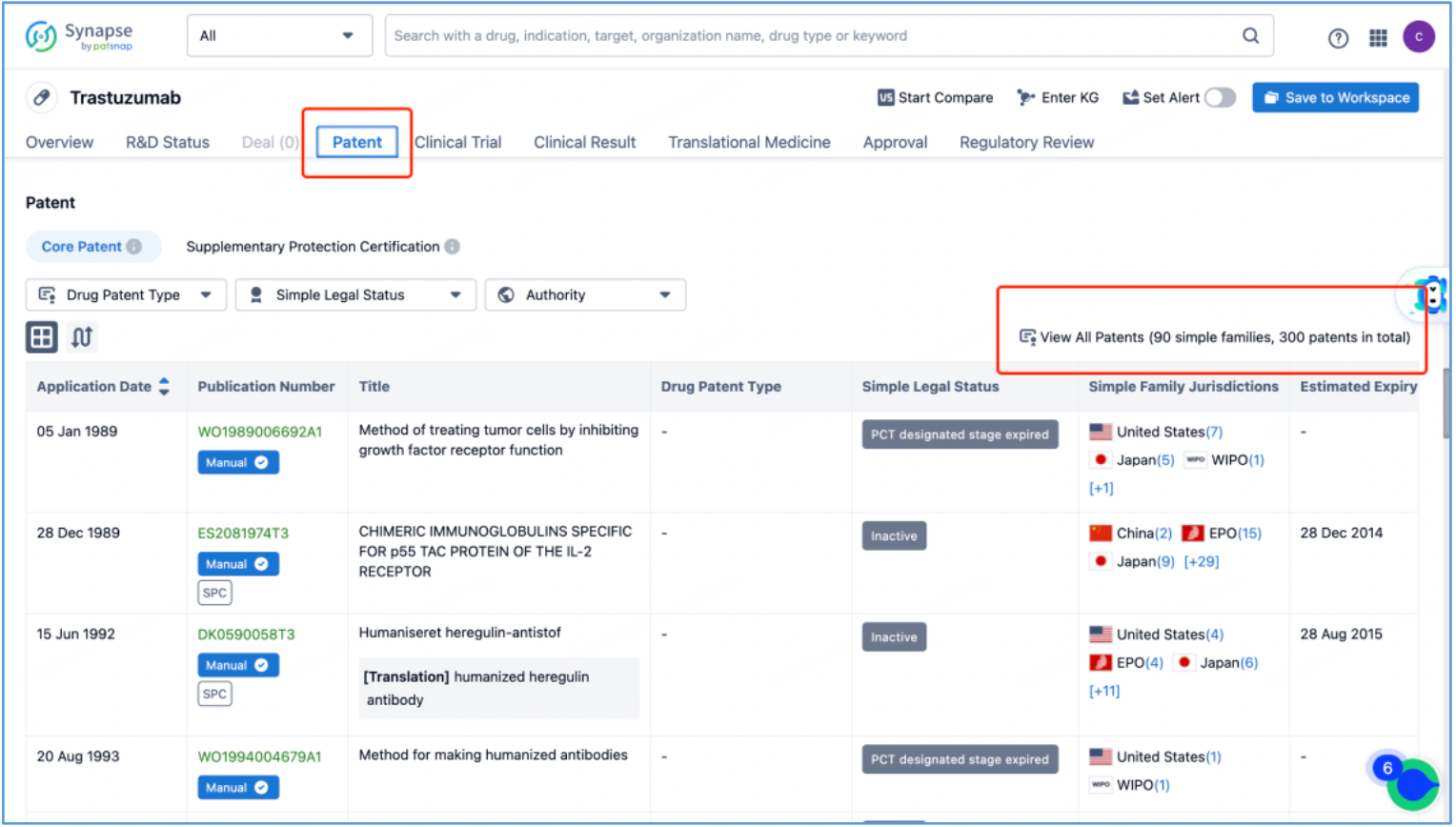
Trastuzumab Antibody
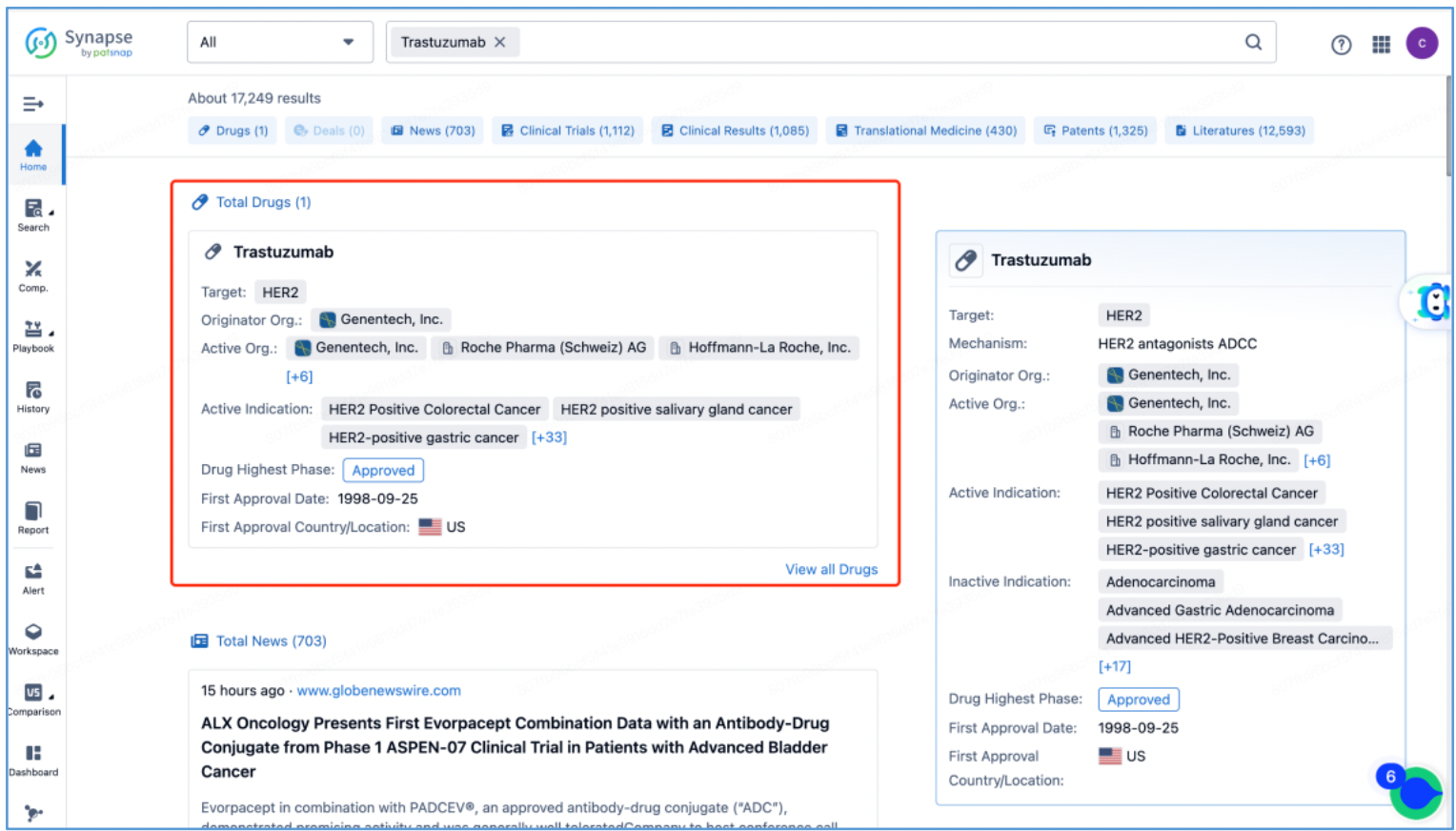
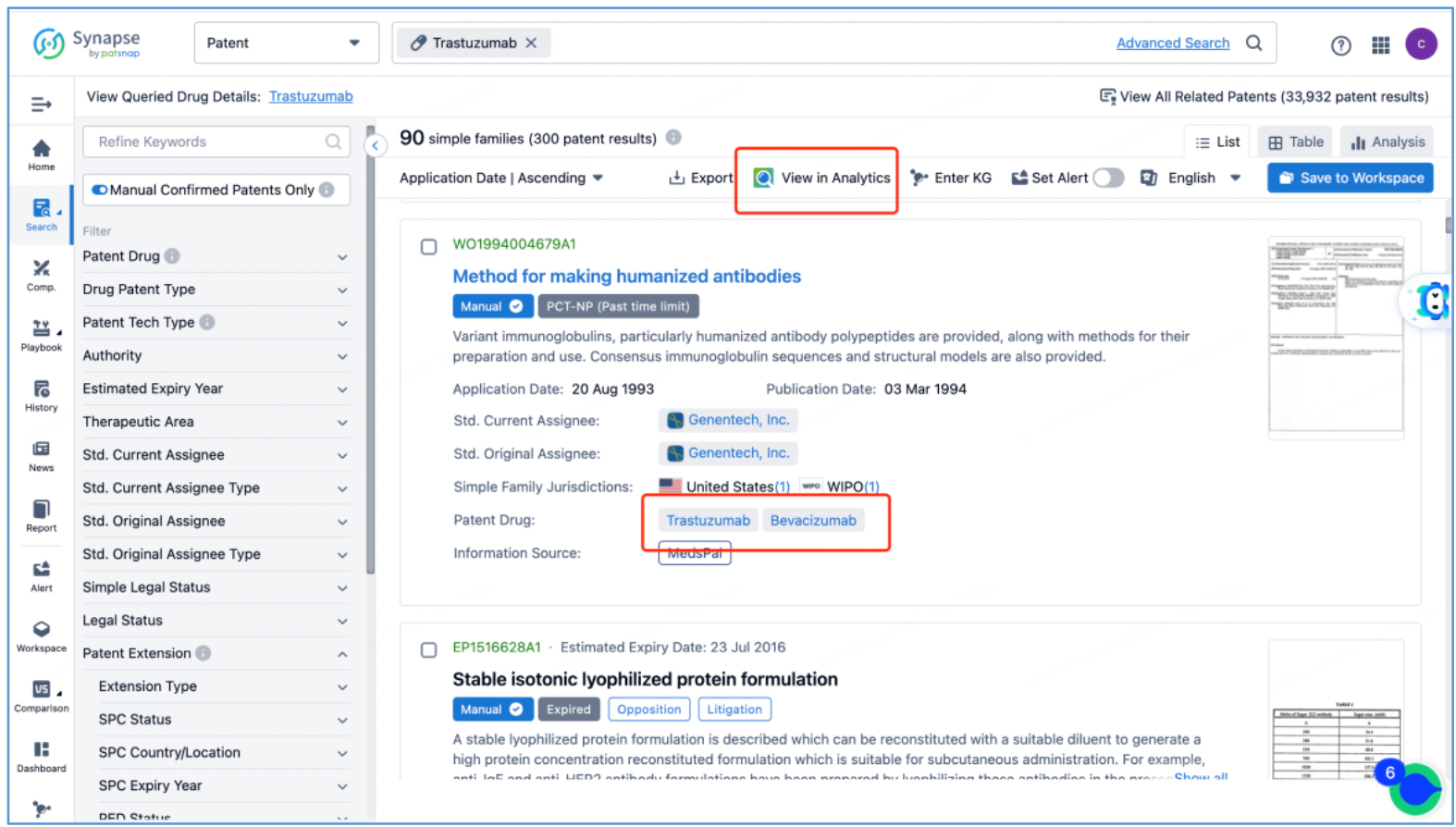
In line with the procedure outlined in section 2.4 for identifying the fundamental product patents of DS-8201, an investigation is conducted to ascertain the core product patents of the trastuzumab antibody by first searching the drug in Patsnap Synapse, and then viewing its related patents directly in Analytics. By meticulously examining the claims and descriptions of each patent, it is determined that the original product patent for trastuzumab in the United States is US5821337A (family patent of PCT application WO1994004679A1), which is then saved to Workspace. Moreover, the sequence information provided in this patent, including CDR, heavy chain, and light chain sequence data, can be compared to the sequence information obtained in section 2.2 to further validate the accuracy of the search.
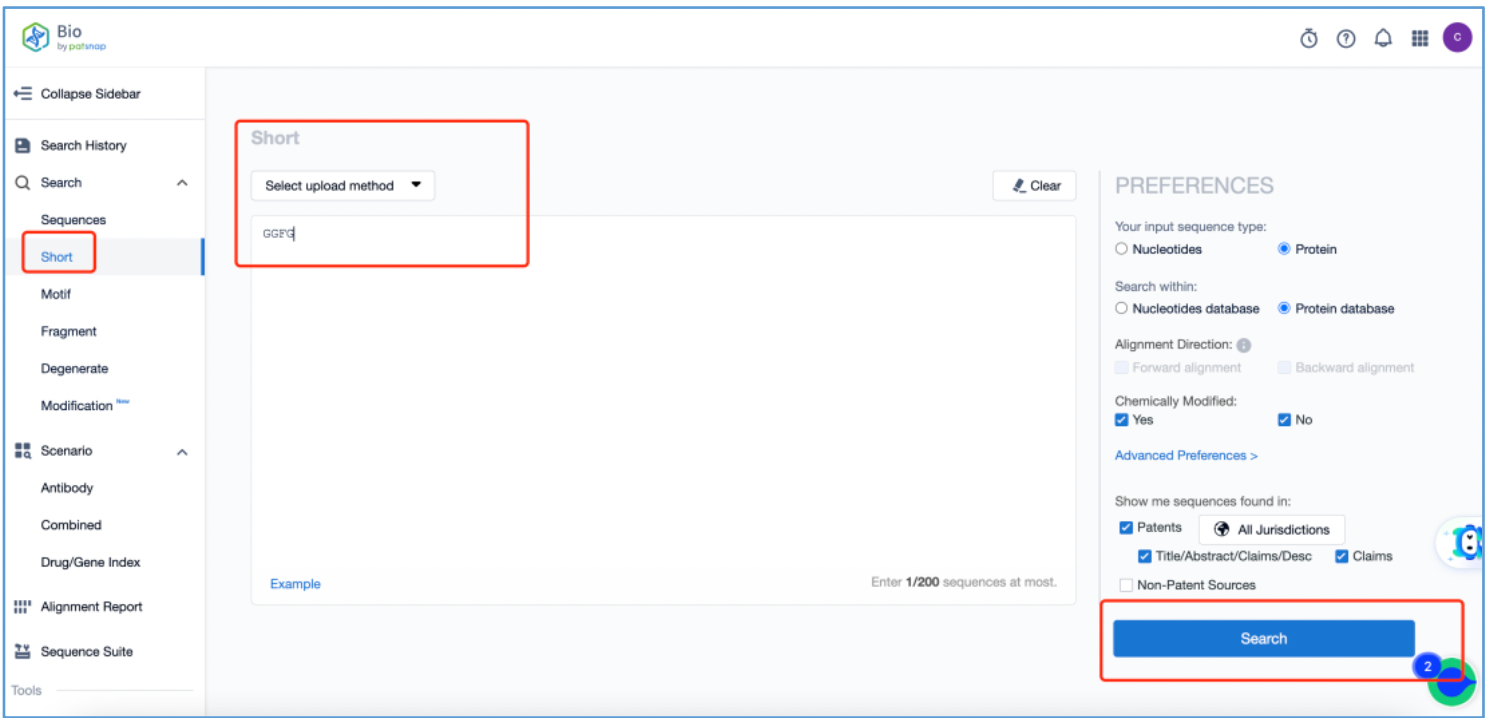
Tetrapeptide Linker
The tetrapeptide linker possesses the ability to be recognized and cleaved by specific proteases within tumors, thus releasing toxin molecules. To analyze the original patents for the linker, log in to Patsnap Bio and navigate to the short sequence search tool. Input the initial linker sequence "GGFG" that was identified in section 2.2 and click search.
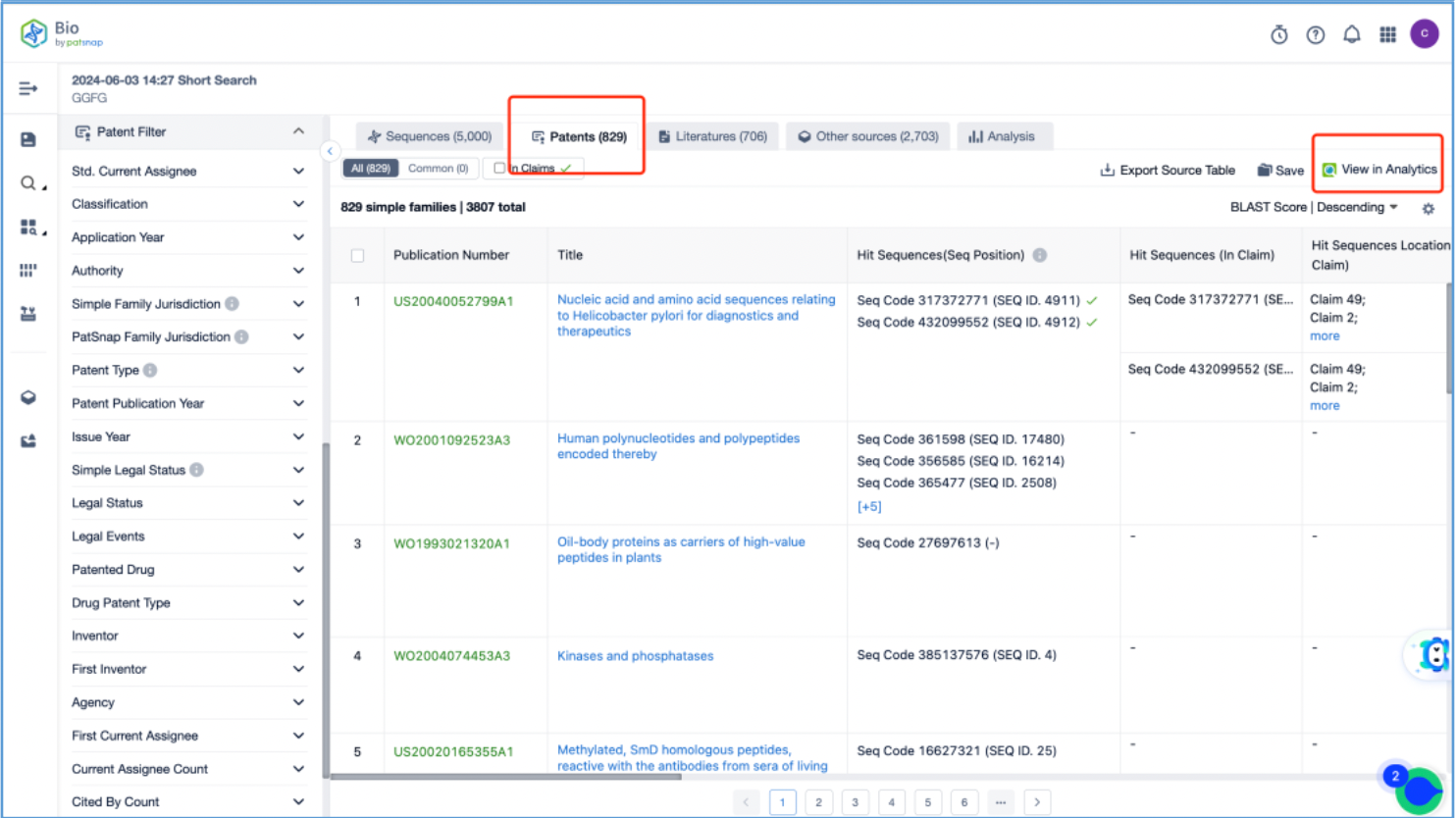
Open the patent results tab and click to "View in Analytics". In Analytics, sort the results by application date, arranging them from the oldest to the latest. To quickly identify the original patent for the linker, focus on the claims of the relevant patents that exhibit a 100% sequence alignment, located in the third column. With this approach, it is determined that the original US patent for the GGFG tetrapeptide linker is US6436912B, which is then saved to Workspace.
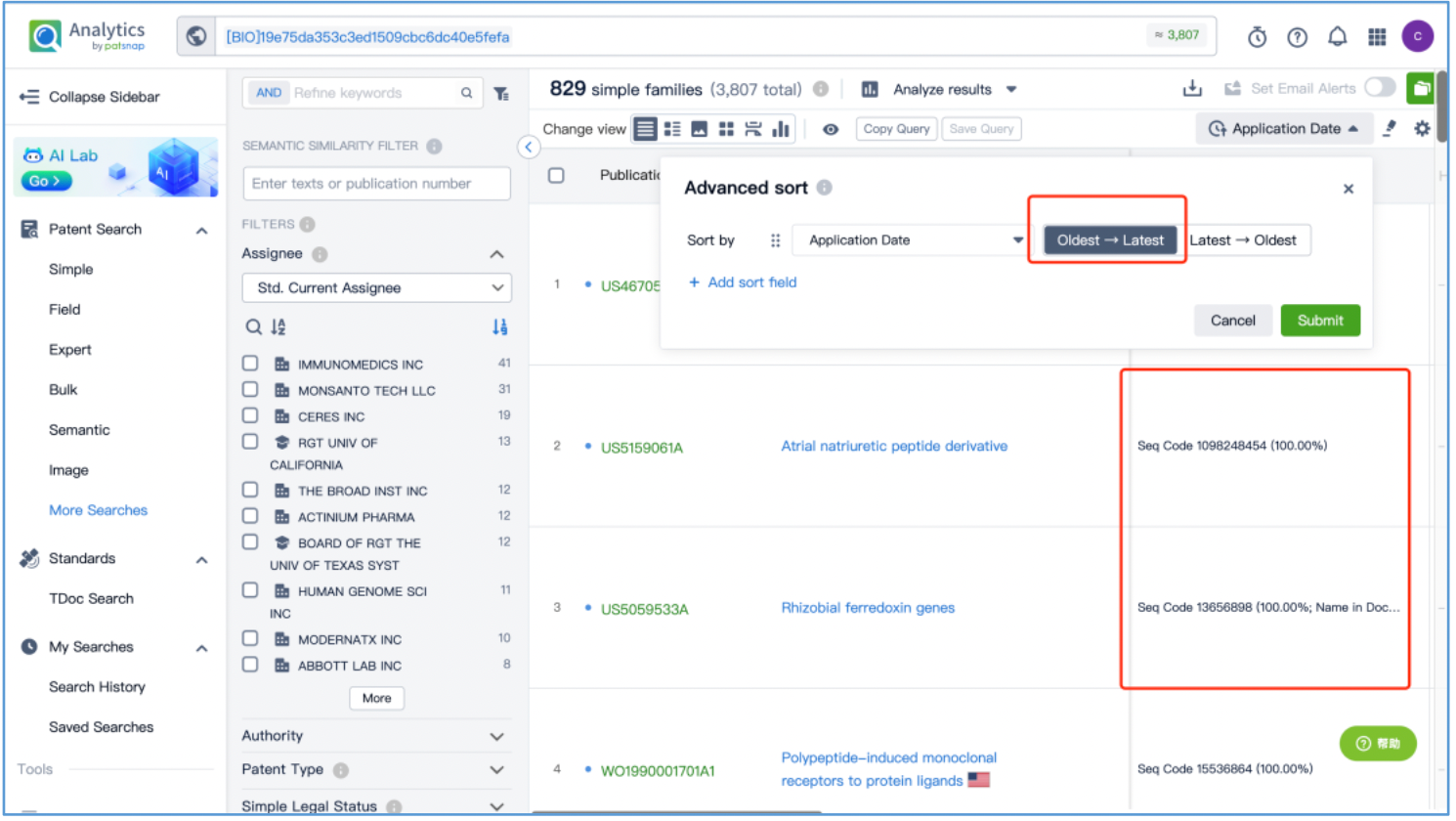
As demonstrated by this search, it is apparent that when dealing with multi-component drugs such as ADCs, the core patents acquired from Patsnap Synapse Database may not encompass the original patents for each individual component. Therefore, it is essential to search for the original patents specific to each component based on their structural characteristics, using the appropriate databases. In this case, a structure search in Patsnap Chemical was used to investigate toxin DX8591, a drug search in Patsnap Synapse was used to investigate the trastuzumab antibody, and a short sequence search Patsnap Bio was used to investigate the linker. By securing the original patents for each component individually, the patent analysis for the drug can be further refined.
Analyzing All Patents Related to DS-8201 and Creating a Patent Layout Diagram
In this phase, all the patents that have been compiled within the "DS-8201" workspace are thoroughly analyzed. The analysis can be carried out by either accessing and reviewing the patents online within the Workspace, or by exporting all the patents for offline examination. By thoroughly examining the claims and descriptions of the patents within the Workspace, with a focus on geographical regions, subject matters of protection, patent legal status, and application dates, two patent layout diagrams are generated for DS-8201, organized by application timeline in the US and China.
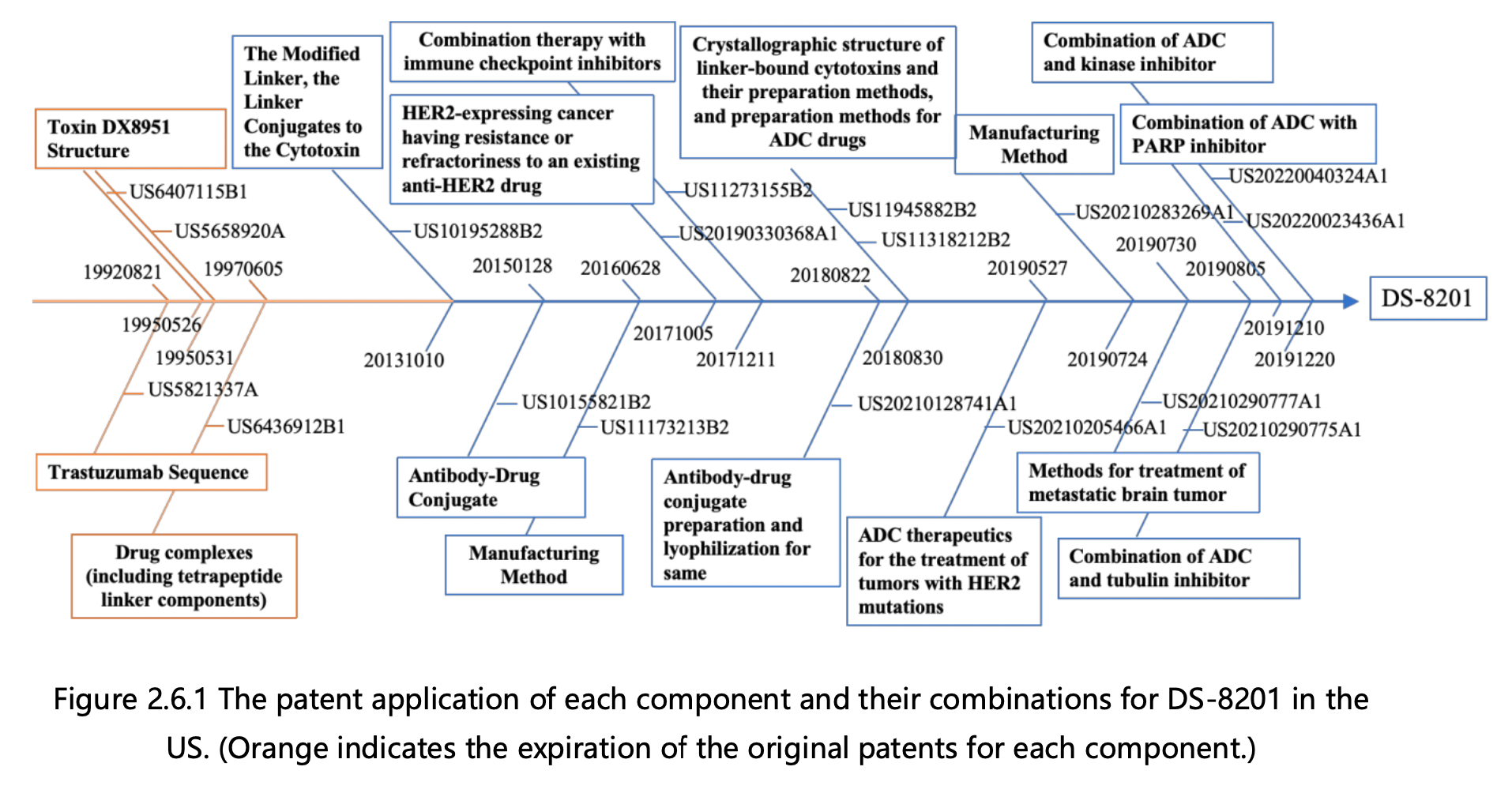
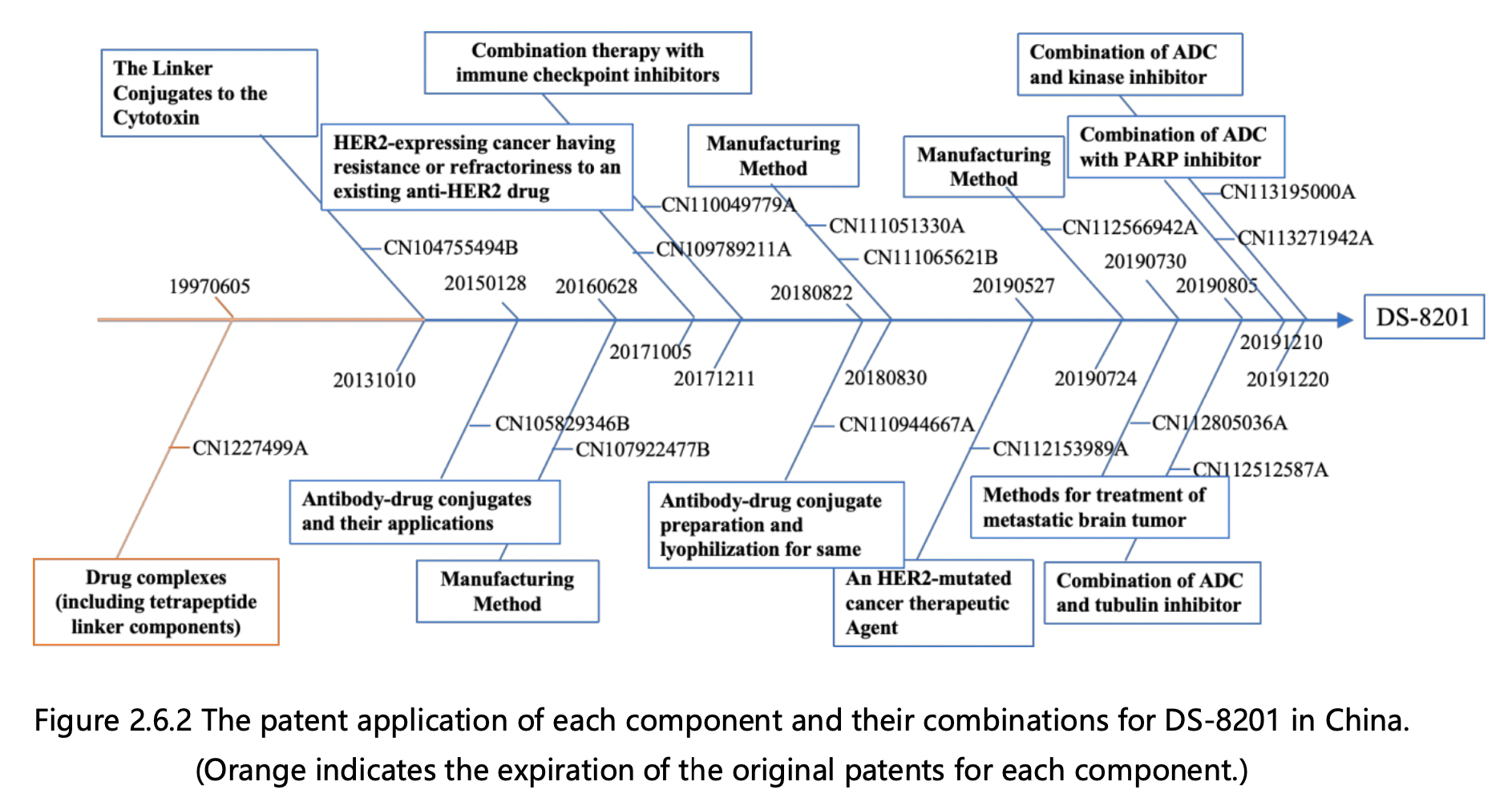
Figures 2.6.1 and 2.6.2 can be used to deduce the overall patent protection status of DS-8201, as well as the specific protection status for each component, in the United States and China. The key findings are as follows:
1. Trastuzumab Protection: The initial patent application for protecting trastuzumab was filed in 1992 by Genentech, the original patent owner. In 2009, Genentech was acquired by Roche, resulting in a change of patent ownership to Roche Pharmaceuticals. This patent has now expired. Notably, the original patent for trastuzumab did not extend to China, leading to the absence of a corresponding application in that jurisdiction. Trastuzumab was launched in the US in 1998 and achieved remarkable success, with peak global sales of $6.8 billion. It consistently ranked among the top three drugs in global sales, offering renewed hope to numerous breast cancer patients.
2.Tetrapeptide Linker Protection: The fundamental patent for safeguarding the tetrapeptide linker was filed in 1997 by Daiichi Sankyo, the patent owner. This patent has now expired. Although the patent was granted in the US, it was not authorized in China, rendering it invalid in that jurisdiction.
3. Toxin DX8951 Protection: The core patents for protecting toxin DX8951 were filed in 1995, including a compound Markush patent and a specific compound patent, both owned by Daiichi Sankyo. These patents have also expired. Similar to the tetrapeptide linker, the basic patents for toxin DX8951 did not enter the mainland China market, resulting in the absence of a corresponding application in that jurisdiction.
4.Modified Tetrapeptide Linker and DX8951 Linker-Payload Protection: The foundational patent for protecting the modified tetrapeptide linker and DX8951 Linker-Payload was filed in 2013 by Daiichi Sankyo and is currently under protection. This patent has been authorized in both the US and China.
5.DS-8201 Protection: The primary patent for protecting DS-8201 itself was filed in 2015 by Daiichi Sankyo and has been authorized. The basic patent for DS-8201 has been granted in both the US and China and remains under protection. DS-8201 exhibits effectiveness not only against HER2-high expressing tumors, where trastuzumab is effective, but also against some HER2-low expressing tumors that do not respond well to trastuzumab treatment. Additionally, it has shown efficacy in treating brain metastases. Approximately 15% of breast cancer patients are HER2-positive, while the majority are HER2-negative, with 45-55% classified as HER2-low expressing. Trastuzumab and pertuzumab treatments have proven ineffective for the large HER2-low expressing patient group, but DS-8201 offers new hope to these individuals.
6.Derivative Patents Surrounding DS-8201: This category includes over 10 patent applications for various combination therapies and preparation methods that have been filed since 2016. As depicted in Figures 2.6.1 and 2.6.2, applications in both the US and China have progressed in parallel.
The key US and China patent applications related to DS-8201 are summarized below:
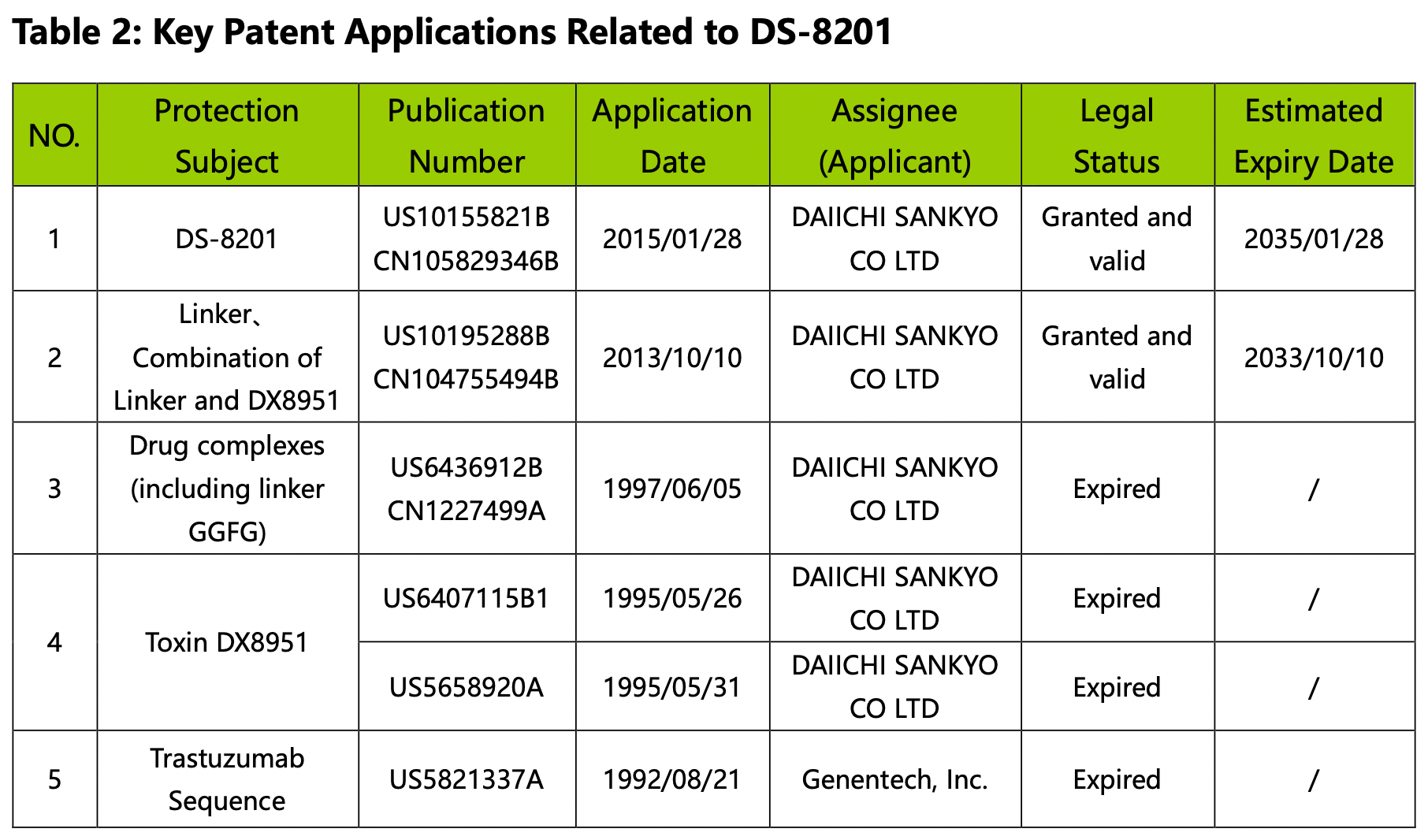
By analyzing Figures 2.6.1, 2.6.2, and Table 2, we gain insights into the historical and current aspects of DS-8201 and the factors influencing its patent landscape. DS-8201 represents a typical A-Linker-B class of ADC drugs.
When it comes to innovative A-Linker-B drugs like DS-8201, patents can be obtained for any of the initial A, B, or Linker components. Moreover, if the A-Linker-B combination itself exhibits distinctive physicochemical properties or notable pharmacological advantages, the novel amalgamation of existing elements can also warrant a new patent. This effectively extends the exclusivity period of the product in the market, emphasizing the importance of mapping the underlying patent application trail to understand the full IP protection strategy of ADC drugs.
This guide offers valuable insights into managing the interplay between original patents for individual ADC components and patents for their combinations. Furthermore, it sheds light on the essential methods for conducting searches for similar drugs.
Summary
This report provides an overview of the patent research process for antibody-drug conjugate (ADC) drugs, with a specific focus on Daiichi Sankyo's DS-8201 drug. The multi-pronged method demonstrated in this guide required the use of various intelligence databases, including Patsnap Analytics, Synapse, Bio, and Chemical, alongside web searches, to successfully retrieve relevant patents, perform core patent analysis, extract patent objects, and conduct supplementary searches for individual drug components. By applying this comprehensive approach, the final DS-8201 patent layout diagrams for the US and China were generated, yielding valuable insights into the drug's development and protection strategy. This guide can be used to similarly assess other ADC drugs, assisting biopharmaceutical companies in infringement analysis, biosimilar development, and strategic investment and financing decisions.
For more information, please click the image link below to access the full report.




