Zai Lab Presents ZL-1218 Phase 1 Results at ESMO 2024: Advancing Anti-CCR8 Therapy in Tumors
Zai Lab Limited (NASDAQ: ZLAB; HKEX: 9688) disclosed that findings from a Phase 1 clinical trial of ZL-1218, their anti-CCR8 antibody, are set to be showcased in a poster session at the European Society for Medical Oncology (ESMO) Congress 2024. The event will be held from September 13-17, 2024, in Barcelona, Spain. The initial outcomes from the ongoing dose-escalation study (NCT05859464) will demonstrate the potential of ZL-1218 to decrease regulatory T cells and influence T-cell activity within the tumor microenvironment (TME) of advanced solid tumors.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
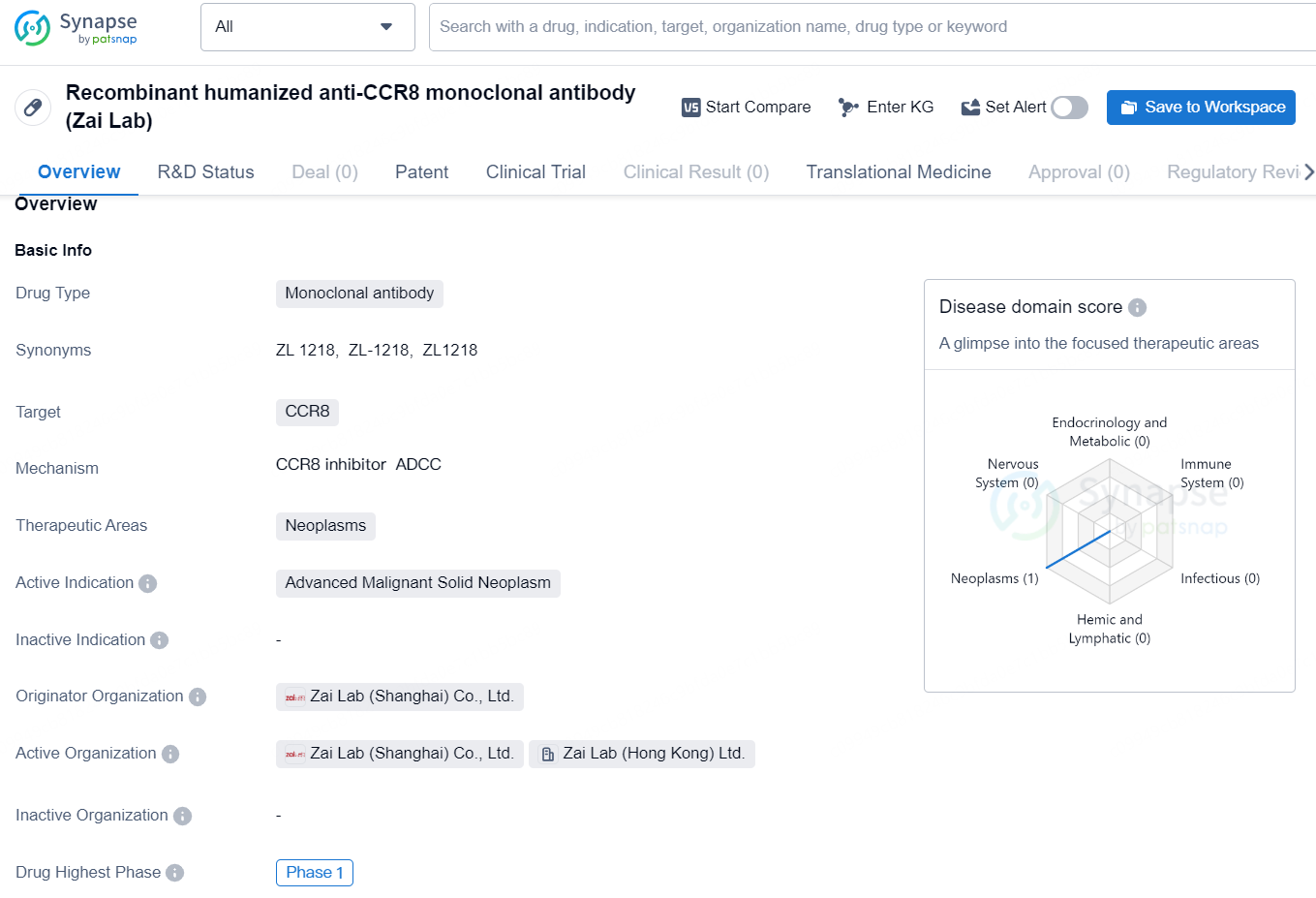
ZL-1218 is a humanized monoclonal antibody designed to target CCR8, a chemokine receptor predominantly found on tumor-associated regulatory T cells (Treg). This antibody contains an enhanced Fc region, which may help bolster the antitumor immune response by eliminating CCR8+ Treg cells through antibody-dependent cellular cytotoxicity. ZL-1218 has the potential to be a ground-breaking treatment for solid tumors. A global Phase 1 clinical trial is currently underway to evaluate ZL-1218 both as a standalone therapy and in synergy with pembrolizumab in patients with advanced solid tumors.
"Addressing the complexities of the tumor microenvironment is essential for advancing patient outcomes in cancer immunotherapy," said Rafael G. Amado, M.D., President and Head of Global Research and Development at Zai Lab. "By reducing the number of suppressive tumor-associated regulatory T cells, ZL-1218 may reignite the immune system’s attack on advanced solid tumors. We are eager to present preliminary findings from our Phase 1 trial at ESMO 2024."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
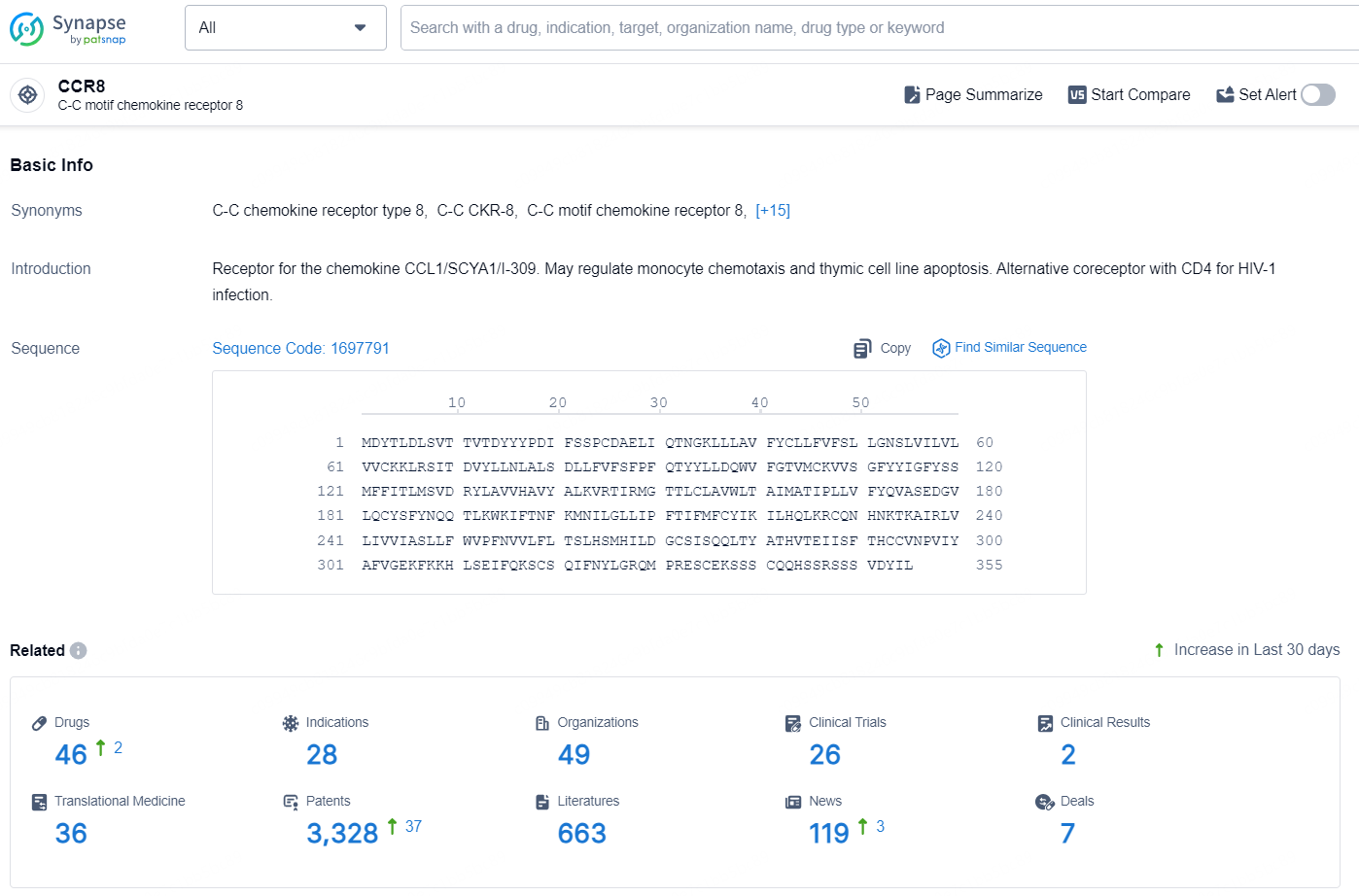
According to the data provided by the Synapse Database, As of August 29, 2024, there are 46 investigational drugs for the CCR8 targets, including 28 indications, 49 R&D institutions involved, with related clinical trials reaching 26, and as many as 3328 patents.
The recombinant humanized anti-CCR8 monoclonal antibody developed by Zai Lab is a type of monoclonal antibody that targets the CCR8 protein. This drug falls in the therapeutic area of neoplasms, with a primary focus on the treatment of advanced malignant solid neoplasms. The originator organization of this drug is Zai Lab (Shanghai) Co., Ltd., which is responsible for its development and potential commercialization.