Pegfilgrastim: A Quick Look at Its R&D Progress and Clinical Results from the 2024 ASCO_GI
The original FOLFIRINOX regimen (5-fluorouracil, leucovorin, irinotecan, and oxaliplatin) for pancreatic ductal adenocarcinoma (PDAC) is associated with a high risk of febrile neutropenia (FN), and pegfilgrastim has been used to reduce the risk. However, it remains unclear how long pegfilgrastim needs to continue. On 18 Jan 2024, the phase II study of the safety and efficacy of discontinuing pegfilgrastim for pancreatic adenocarcinoma treated with FOLFIRINOX was reported in 2024 ASCO_GI.
Pegfilgrastim's R&D Progress
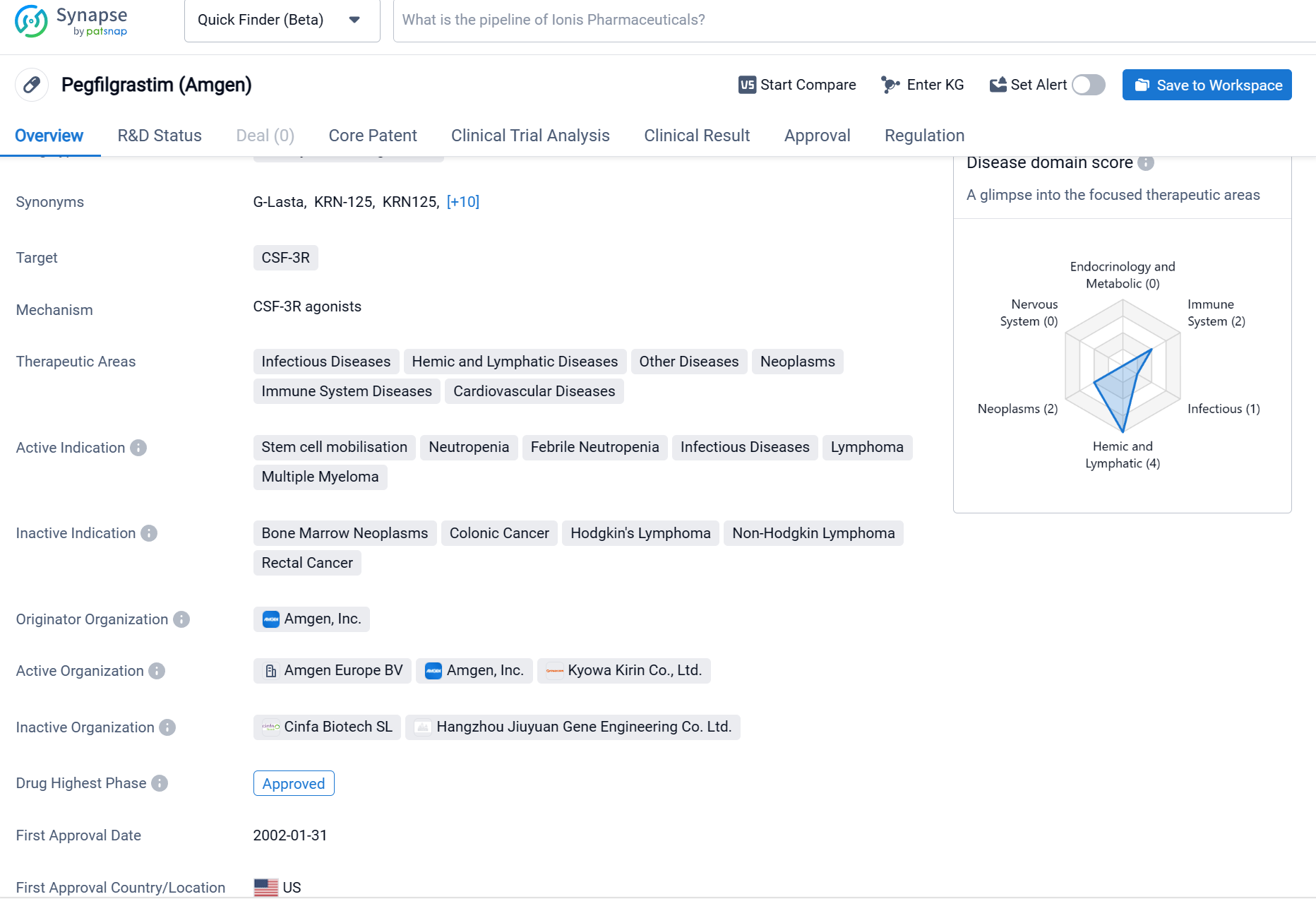
Pegfilgrastim, developed by Amgen, Inc., is a drug classified as a colony-stimulating factor. It targets the CSF-3R receptor and is used in the treatment of various diseases across multiple therapeutic areas. These areas include infectious diseases, hemic and lymphatic diseases, other diseases, neoplasms, immune system diseases, and cardiovascular diseases.
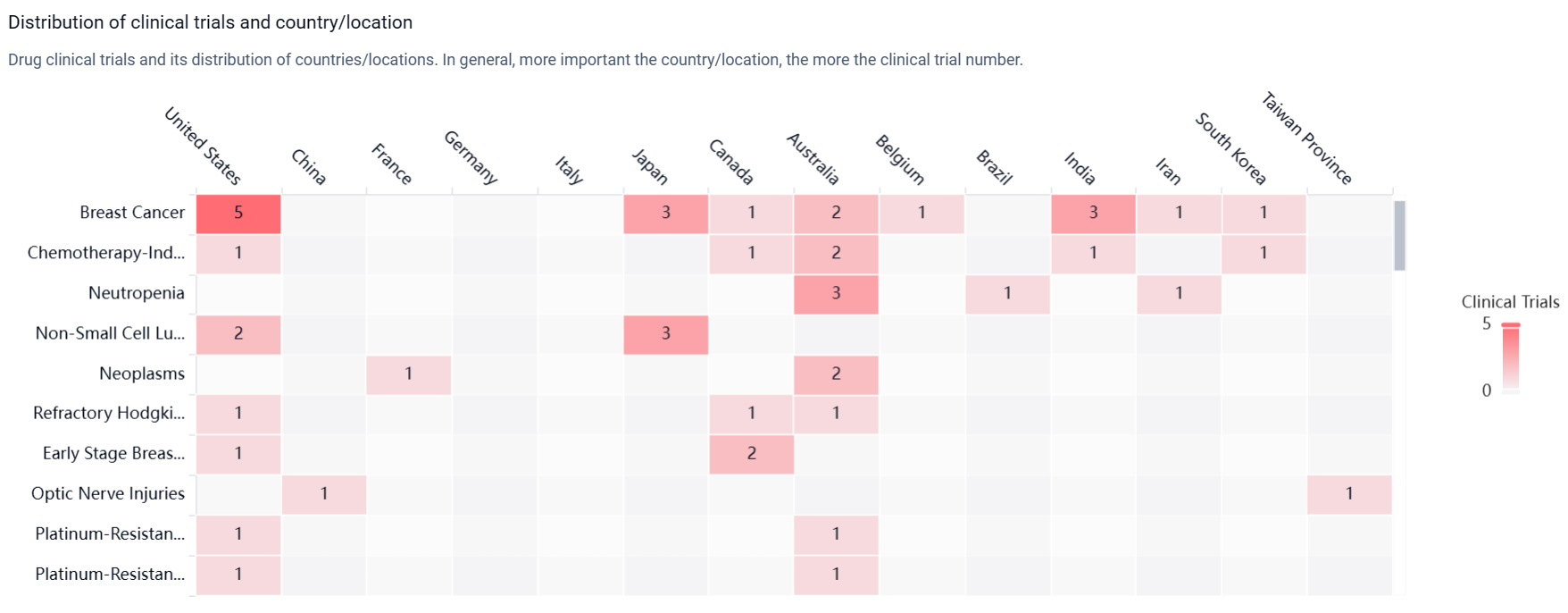
According to the Patsnap Synapse, Pegfilgrastim has achieved approval in several countries globally. Its highest phase of development in China is currently pending. And the clinical trial distributions for Pegfilgrastim are primarily in the United States, China and France. The key indication is Breast Cancer.
Detailed Clinical Result of Pegfilgrastim
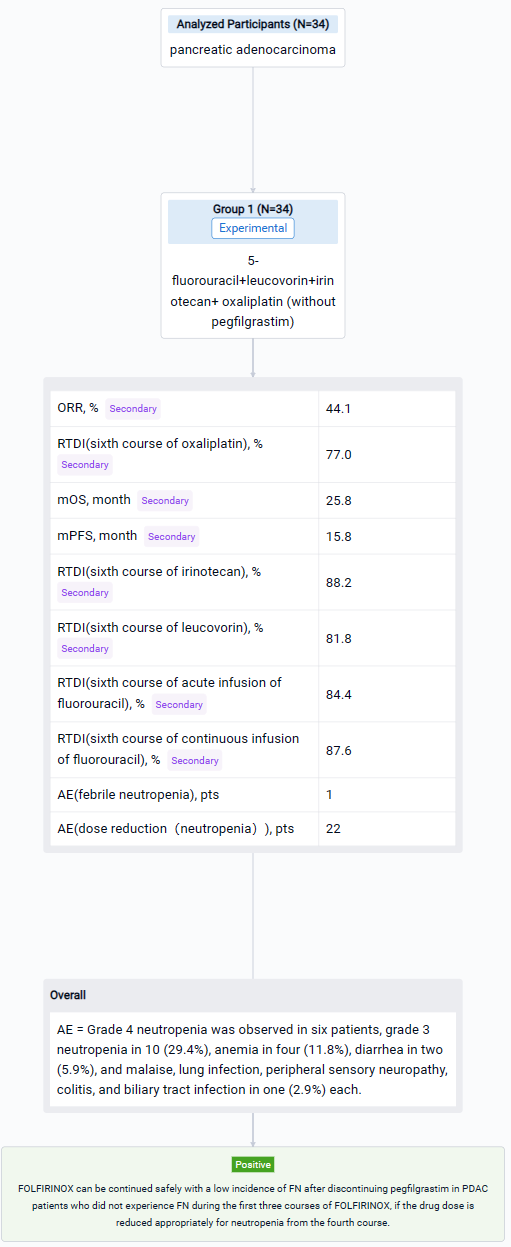
This Non-Randomized, Single Group Assignment clinical trial (JPRN-UMIN000028055) was aimed to evaluate the safety and efficacy of FOLFIRINOX after discontinuing pegfilgrastim in PDAC patients.
In this study, patients with PDAC who received the first three courses of FOLFIRINOX, with primary pegfilgrastim prophylaxis for FN, and did not experience FN were included. The patients continued on FOLFIRINOX without pegfilgrastim from the fourth course. The primary endpoint was development of FN. The secondary endpoints included relative dose intensity (RDI), objective response rate (ORR), progression-free survival (PFS), and overall survival (OS). The threshold and expected development of FN were 20% and 7%, respectively, at a one-sided significance level of 0.10 and statistical power of 80%. Based on this hypothesis, we calculated the number of patients needed to be 33.
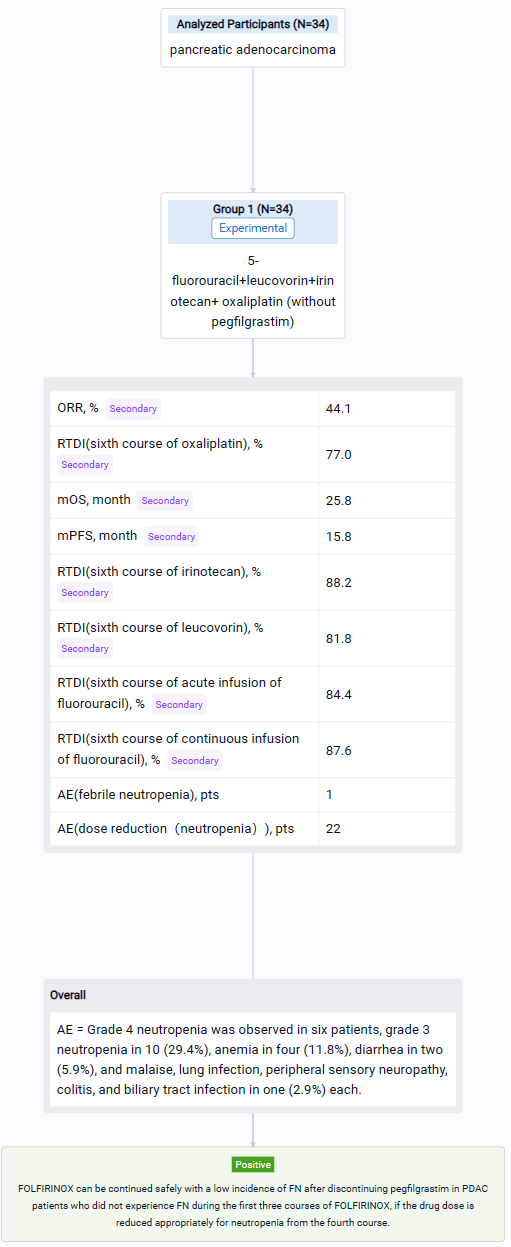
The result showed that in total, 34 patients (20 male, median age 66 years) were enrolled from August 2017 to September 2021. Twenty-three patients were unresectable, and 11 were borderline resectable. FN developed in one patient. Grade 4 neutropenia was observed in six patients, grade 3 neutropenia in 10 (29.4%), anemia in four (11.8%), diarrhea in two (5.9%), and malaise, lung infection, peripheral sensory neuropathy, colitis, and biliary tract infection in one (2.9%) each. Twenty-two patients required dose reduction, mainly because of neutropenia. Mean RDIs at the sixth course of oxaliplatin, irinotecan, leucovorin, acute infusion of fluorouracil, and continuous infusion of fluorouracil were 77.0%, 88.2%, 81.8%, 84.4%, and 87.6%, respectively. ORR, median PFS, and median OS were 44.1%, 15.8 months, and 25.8 months, respectively.
It can be concluded that FOLFIRINOX can be continued safely with a low incidence of FN after discontinuing pegfilgrastim in PDAC patients who did not experience FN during the first three courses of FOLFIRINOX, if the drug dose is reduced appropriately for neutropenia from the fourth course.
How to Easily View the Clinical Results Using Synapse Database?
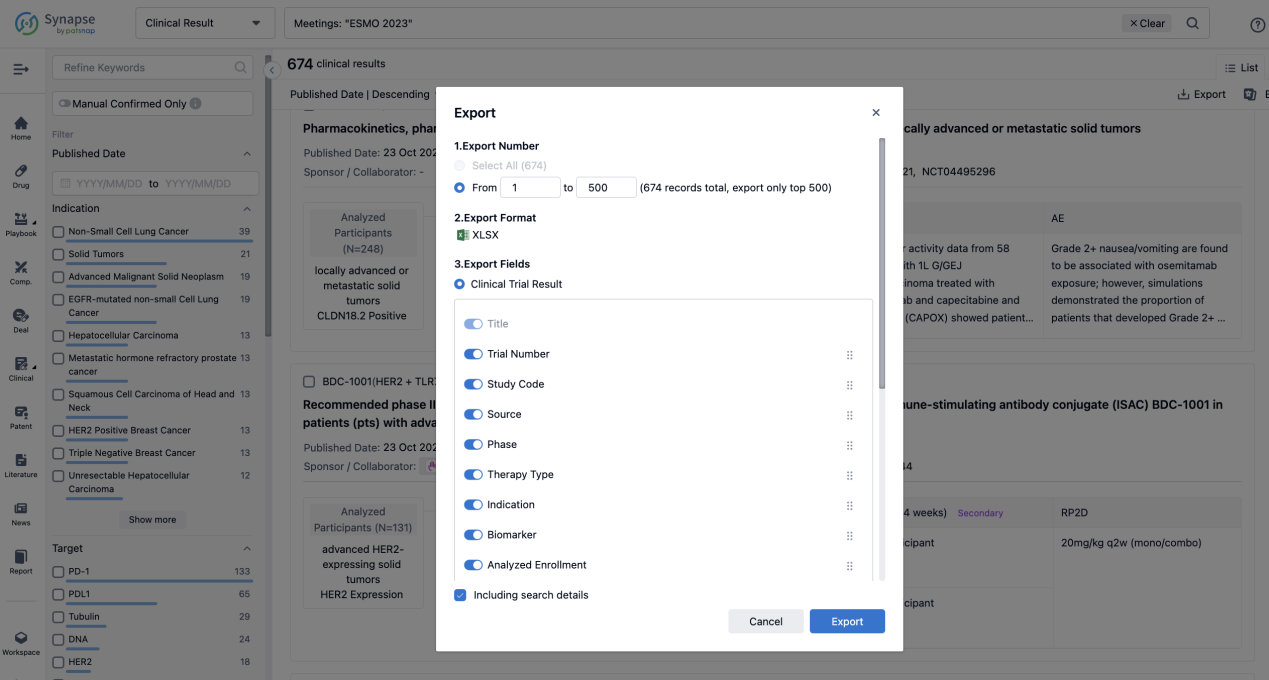
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
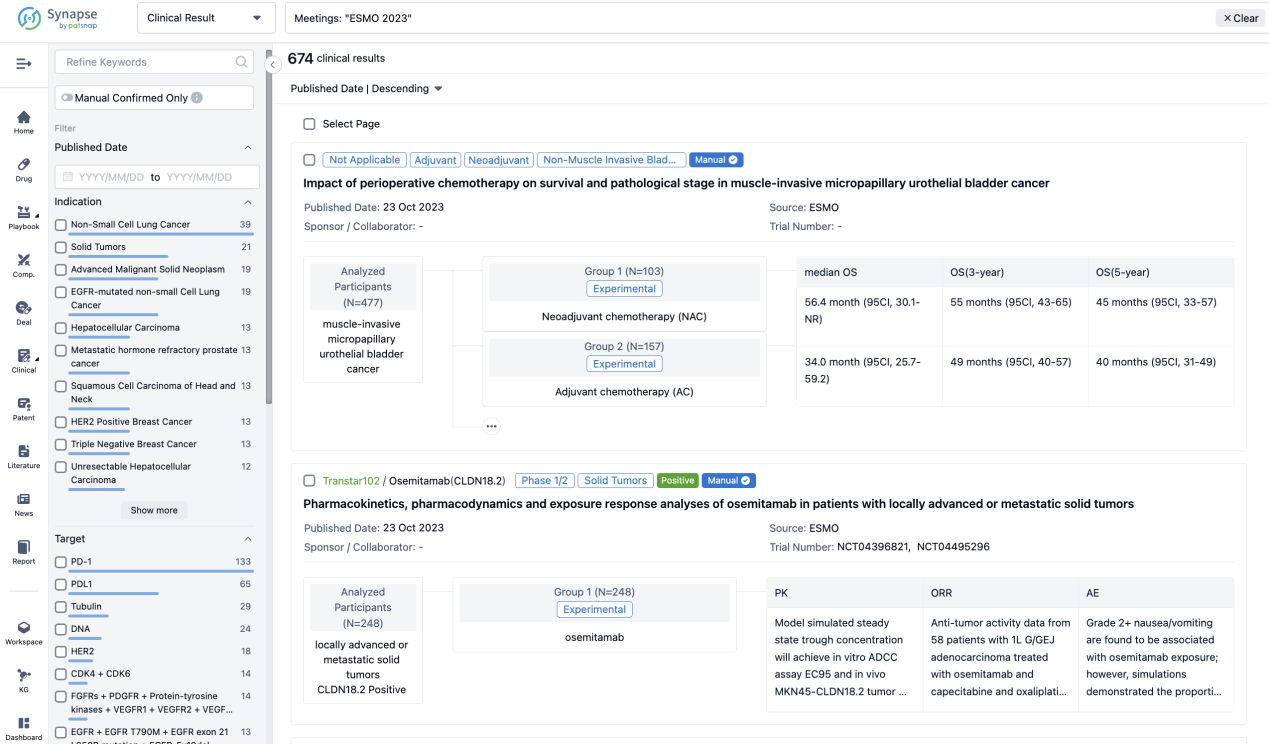
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
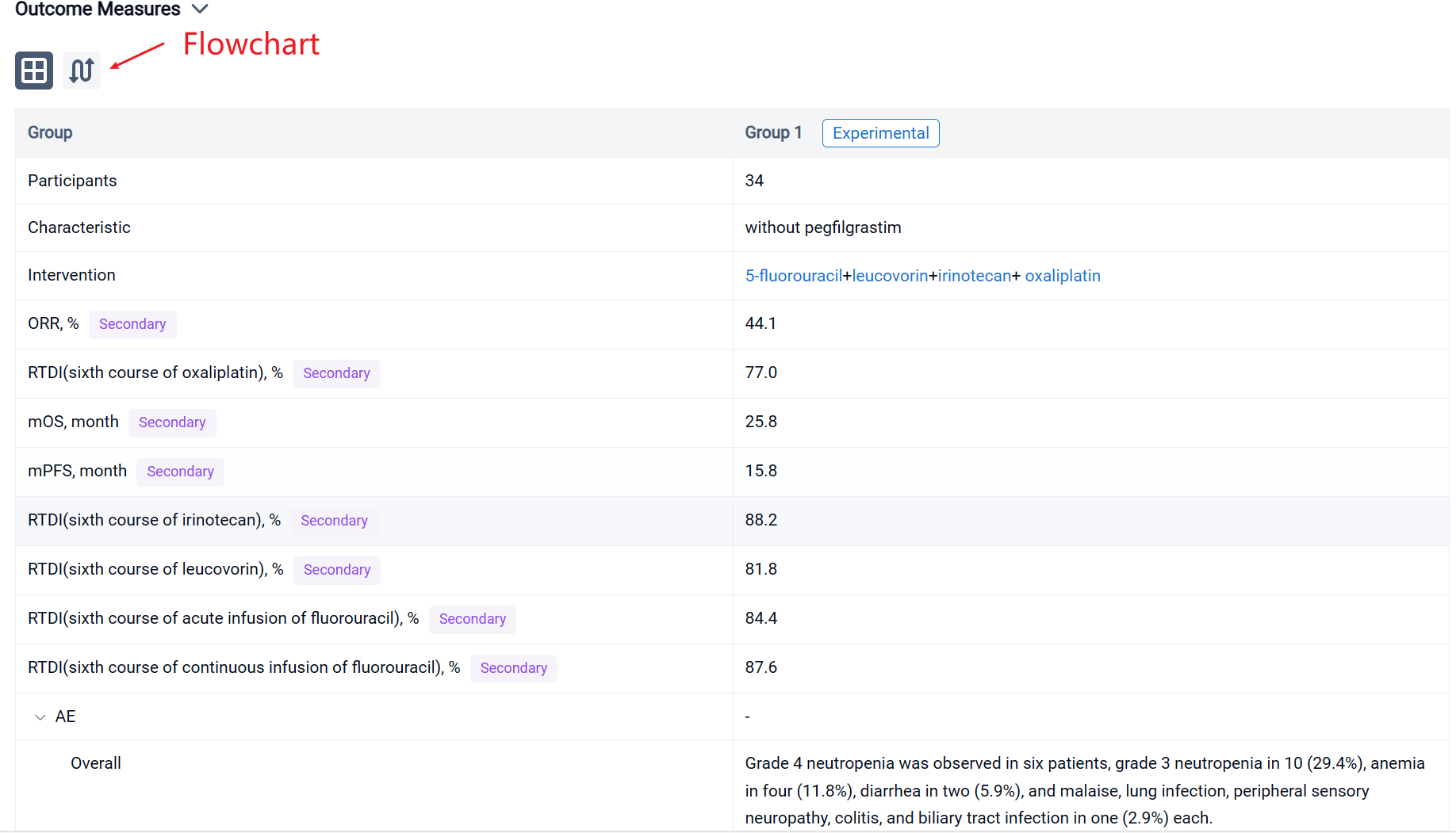
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!