US FDA & Australian Health Authorities Approve ETX101, Encoded Therapeutics' Potential Genetic Treatment for Dravet Syndrome
Encoded Therapeutics Inc., a biotech firm specialized in creating gene-based treatments for critical neurological conditions, has announced its international advancement plan for its primary gene therapy product, ETX101. This therapy is specifically aimed at combating SCN1A-positive Dravet syndrome. Patients suffering from Dravet syndrome, a prevalent form of developmental and epileptic encephalopathy, endure a range of symptoms, such as seizures that do not respond to medication and a halt in neurological progress.
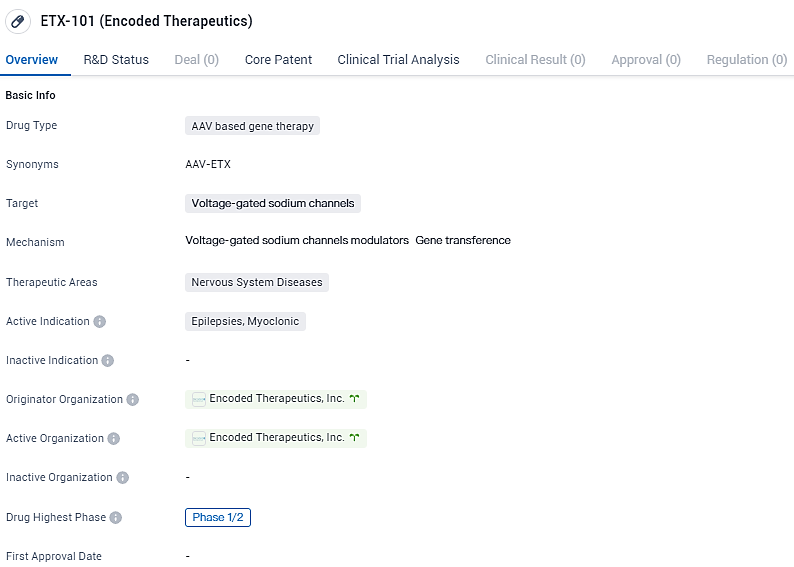
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
In the majority of Dravet syndrome instances, which exceed 85%, the condition is mainly attributable to inactivating mutations within the SCN1A gene. ETX101 is a therapeutic compound involving AAV9 that aims to increase SCN1A gene activity specifically in GABAergic inhibitory neurons, which could potentially target the core issue of the affliction.
Encoded Therapeutics has secured the green light for its ETX101 Experimental New Drug submission from the U.S. FDA and has gained consent through the Clinical Trial Notification process with the Australia's Therapeutic Goods Administration to commence trials for this gene-targeted treatment.
Dr. Joseph Sullivan, a notable expert in Neurology and Pediatrics at UCSF's Pediatric Epilepsy Center of Excellence, expressed optimism that gene therapy might go beyond mere symptom relief to tackle the fundamental cause of Dravet syndrome, heralding a groundbreaking shift in the fight to alleviate the heavy health toll on families affected by the condition.
Later into 2024, Encoded will be divulging more details concerning its research pursuits, which are sharply focused on pinpointing treatments for both uncommon genetic ailments and more widespread diseases, as well as the ongoing clinical assessments involving ETX101 specifically tailored for Dravet syndrome.
As expressed by Kartik Ramamoorthi, Ph.D., a Co-Founder and CEO, Encoded is dedicated to pioneering genetic therapies that harbor the promise and new opportunities for patients grappling with serious CNS conditions. The commencement of clinical testing for Dravet syndrome and the forward movement of our CNS-focused research signifies a strategic step for Encoded, setting the stage for key achievements of our programs in 2024. The company is poised to bring additional updates to the forefront in the upcoming time frame.
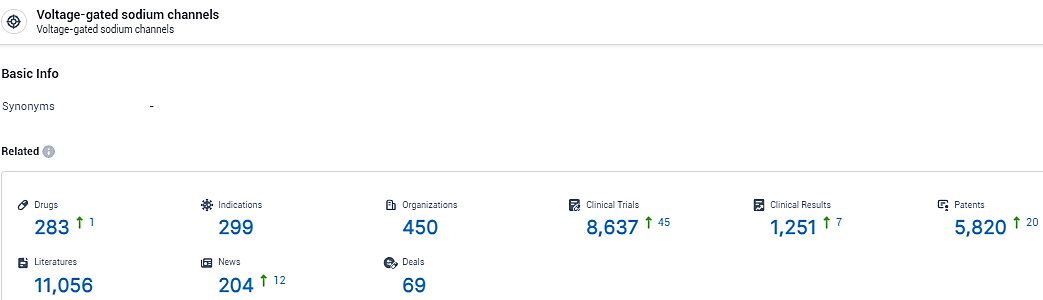
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of February 18, 2024, there are 283 investigational drugs for the Voltage-gated sodium channels target, including 299 indications, 450 R&D institutions involved, with related clinical trials reaching 8637, and as many as 5820 patents.
ETX-101 targets voltage-gated sodium channels and is being developed for the treatment of nervous system diseases, specifically myoclonic epilepsy. ETX101 has been granted Orphan Drug Designation and Rare Pediatric Disease Designation by the US Food and Drug Administration and Orphan Designation by the European Medicines Agency. Currently in Phase 1/2 of development, ETX-101 shows potential as a novel therapeutic option for patients with this indication, although further research is needed to establish its safety and effectiveness.