PepGen Announces Promising Low-Dose Results in Phase 2 CONNECT1-EDO51 Trial for Duchenne Muscular Dystrophy Treatment
PepGen Inc., a biotech company at the clinical stage that aims to revolutionize the treatment of severe neuromuscular and neurological diseases through advanced oligonucleotide therapies, disclosed encouraging clinical results from the initial dose group of PGN-EDO51, their primary investigational asset for individuals with Duchenne muscular dystrophy who have mutations suitable for exon 51-skipping therapy.
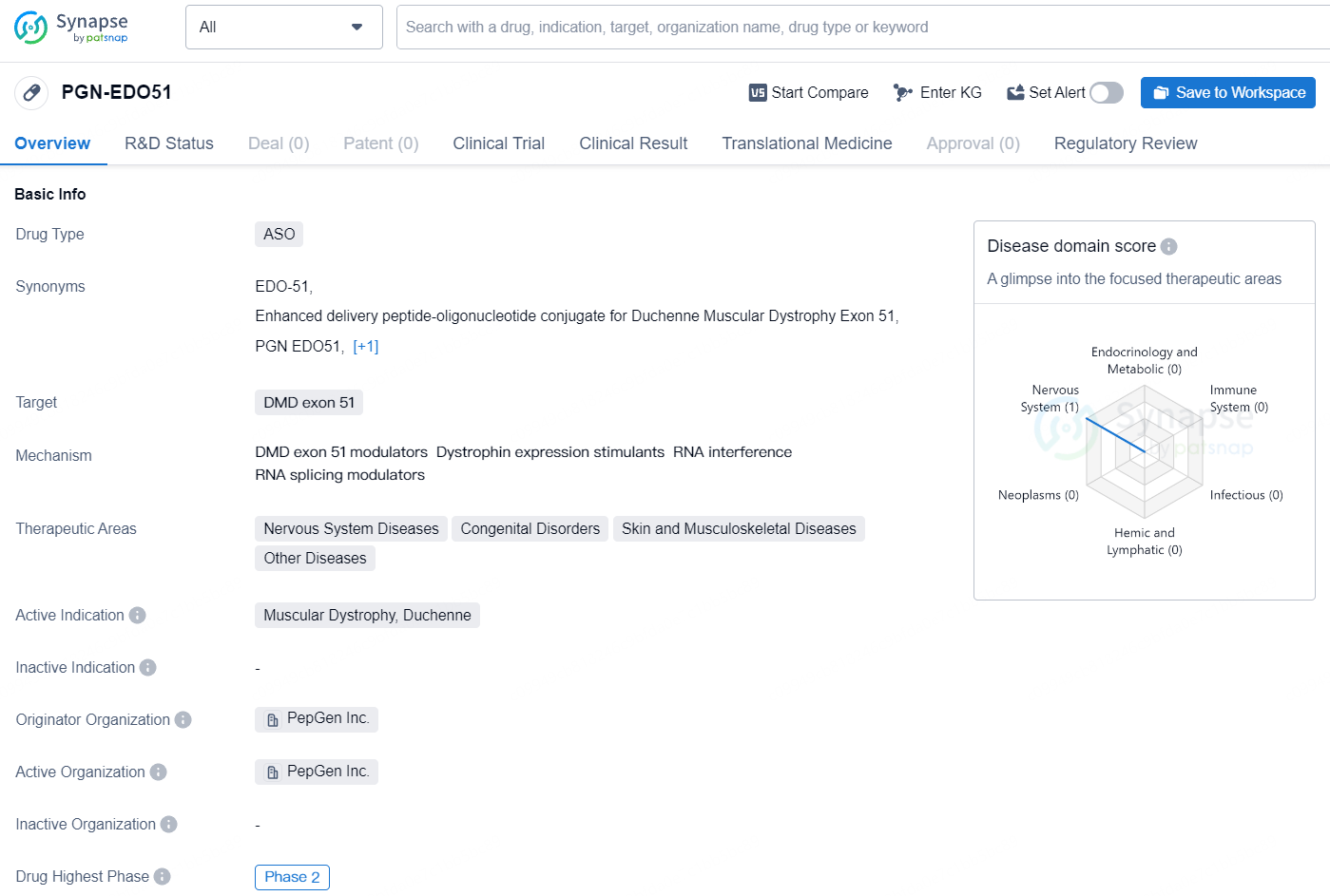
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
In the ongoing Phase 2 CONNECT1-EDO51 trial, PGN-EDO51 exhibited higher rates of exon skipping compared to previous studies using similar PMO dose levels in DMD patients. The company also announced that changes in total dystrophin production and muscle-adjusted dystrophin production from baseline were on par with or surpassed findings from studies involving other oligonucleotide therapies at similar PMO dose levels in DMD patients. Today at 4:30 p.m. ET, the company will hold a conference call with lead investigator Dr. Hugh McMillan from the CONNECT1 trial to discuss the presented data.
"We are pleased with the initial results from our CONNECT1 trial testing PGN-EDO51 in DMD patients. Within three months, the starting monthly dosage of 5 mg/kg resulted in significant exon skipping and increased dystrophin levels in all patients. PGN-EDO51 demonstrated notably higher levels of exon skipping transcripts with lower dosages and in a shorter timeframe compared to other exon 51 therapies currently approved or in development. This suggests that our Enhanced Delivery Oligonucleotide technology is effectively delivering higher amounts of oligonucleotides to the cell nuclei," noted James McArthur, Ph.D., President and CEO of PepGen.
McArthur went on to state, "Importantly, PGN-EDO51 has shown a promising safety profile, supporting our continued evaluation of the 10 mg/kg monthly dosage group in CONNECT1. We intend to utilize the early findings from CONNECT1 to optimize our Phase 2 CONNECT2-EDO51 trial. Based on these initial findings, we are optimistic about the potential for increased dystrophin production levels in the 10 mg/kg cohort of CONNECT1. We eagerly await the results from the first cohort of our placebo-controlled global trial, CONNECT2."
exon 51 is designed to skip exon 51 of the dystrophin transcript, a known therapeutic target for about 13% of DMD patients, with the goal of reinstating the correct reading frame and facilitating the production of a truncated yet functional dystrophin protein. The U.S. Food and Drug Administration has granted PGN-EDO51 Orphan Drug and Rare Pediatric Disease Designations for treating patients with DMD who could benefit from an exon-51 skipping strategy.
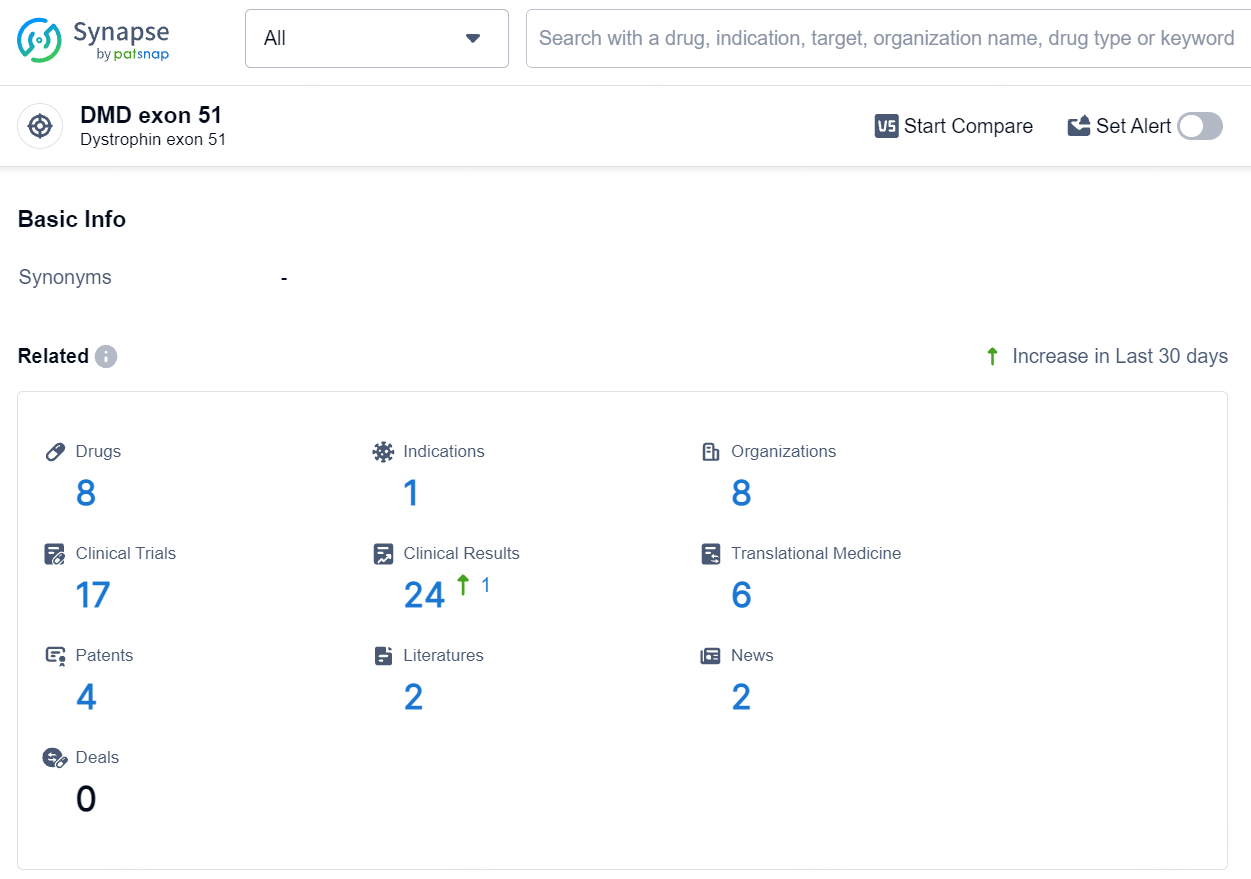
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of August 2, 2024, there are 8 investigational drugs for the DMD exon 51 target, including 1 indication, 8 R&D institutions involved, with related clinical trials reaching 17, and as many as 4 patents.
PGN-EDO51 represents a significant advancement in the development of therapies for DMD and holds promise for improving the quality of life for patients affected by this condition. Its progression to Phase 2 and the regulatory designations it has received further underscore its potential to address the medical needs of a specific patient population. As the drug continues through the development process, it will be important to monitor its safety and efficacy profile, as well as its potential for broader application in related therapeutic areas.