Pfizer's Clinical Advancements with Drug PF-08046052 Reported by LAVA
LAVA Therapeutics N.V., an enterprise dedicated to advancing immuno-oncology through clinical trials with an emphasis on its proprietary Gammabody® technology, which involves dual-specific gamma delta T cell engagers, has recently disclosed that Pfizer has reached a significant clinical advancement goal with the molecule PF-08046052 (formerly SGN-EGFRd2 /LAVA-1223). This achievement has triggered an initial payment of $7 million to LAVA. In September 2022, LAVA provided Seagen with an exclusive global license for the development and commercialization of PF-08046052.
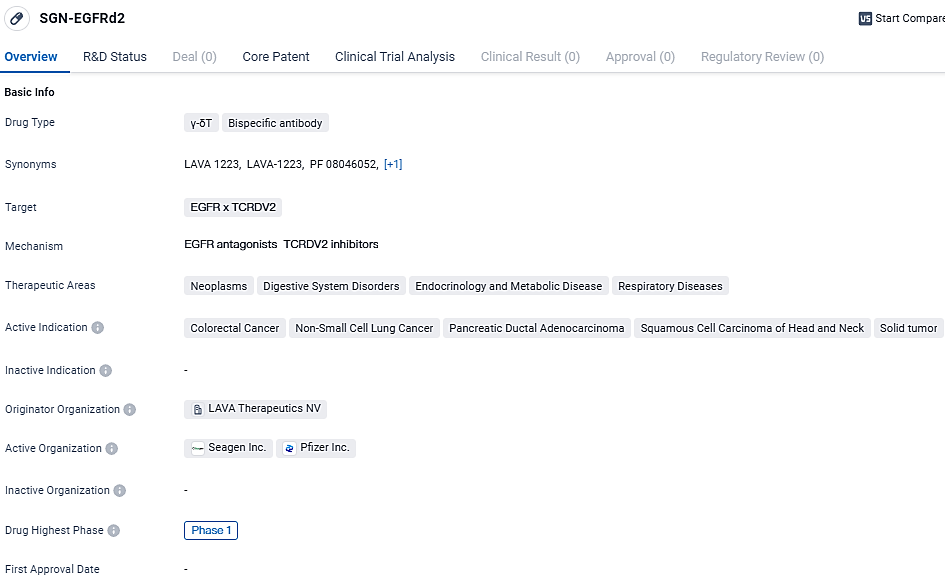
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
It brings us immense satisfaction to acknowledge Pfizer's commencement of clinical trials for PF-08046052, previously known as SGN-EGFRd2/LAVA-1223. We have consistently been optimistic regarding the promise this compound shows in the realm of cancer treatment. This development signifies a crucial advancement toward harnessing the capabilities of the Gammabody® technology at LAVA. As the Phase 1 investigation progresses, we eagerly await further advancements in clinical research and subsequent data revelations," expressed Stephen Hurly, serving as the President and CEO of LAVA.
"The commencement of the Phase 1 trial of PF-08046052 represents the third molecule developed on LAVA's Gammabody® infrastructure to undergo clinical trials, and it will contribute additional insights about tolerability, pharmacological profiling, and potential for combating tumors to the expanding evidence supporting this innovative molecule category," remarked Charles Morris, M.D., Chief Medical Officer at LAVA.
Dr. Morris further noted, "The ongoing developments with PF-08046052 and the PSMA-targeted LAVA-1207, which is currently part of a Phase 1/2a trial, are particularly promising. Both initiatives harness the Vγ9Vδ2 T cells targeted at established markers, which may provide significant preliminary confirmation of the efficacy of LAVA's Gammabody® infrastructure."
PF-08046052 stands as a pioneering candidate from the Gammabody® suite, architected to selectively energize Vγ9Vδ2 T cells upon interaction with the epidermal growth factor receptor (EGFR), fostering a powerful and selective eradication of EGFR-positive cancer cells. EGFR is a distinguished marker expressed profusely in various forms of solid malignancies, such as cancers of the colorectum, lung cells (non-small type), squamous cells in the head and neck region, and pancreatic ductal adenocarcinomas.
Pfizer is at the helm of the current evaluation of PF-08046052 through a Phase 1 trial under an exclusive global licensing contract. As per the stipulations of this arrangement, LAVA has already received an advanced payment of $50 million and stands to gain potential milestone payments which could sum up to the vicinity of $650 million, contingent on the successful passage of development, regulatory compliance, and sales achievements, in addition to receipt of sales royalties.
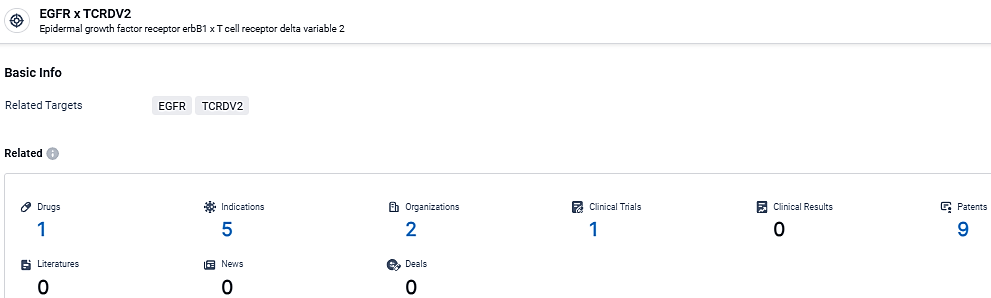
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of March 6, 2024, there are 1 investigational drugs for the EGFR and TCRDV2 target, including 5 indications, 2 R&D institutions involved, with related clinical trials reaching 1, and as many as 9 patents.
PF-08046052 is a promising drug in the field of biomedicine. Its bispecific antibody design and targeting of EGFR and TCRDV2 make it a potentially effective treatment for various types of cancer, including colorectal cancer, non-small cell lung cancer, pancreatic ductal adenocarcinoma, squamous cell carcinoma of the head and neck, and solid tumors. However, further clinical trials are needed to determine its efficacy and safety.