Phanes Therapeutics Partners with Roche for Clinical Trial of PT217 and Anti-PD-L1 Treatment
Phanes Therapeutics, Inc., a biotechnology firm engaged in cutting-edge cancer drug research and advancement, recently declared the initiation of a clinical supply partnership with Roche. Under this agreement, they will conduct research on PT217, a pioneering bispecific antibody that targets DLL3 and CD47. This study will evaluate the combination of PT217 with Roche's anti-PD-L1 therapy, atezolizumab, in treating patients with small cell lung cancer, large cell neuroendocrine carcinoma of the lung, and neuroendocrine carcinomas outside of the lungs.
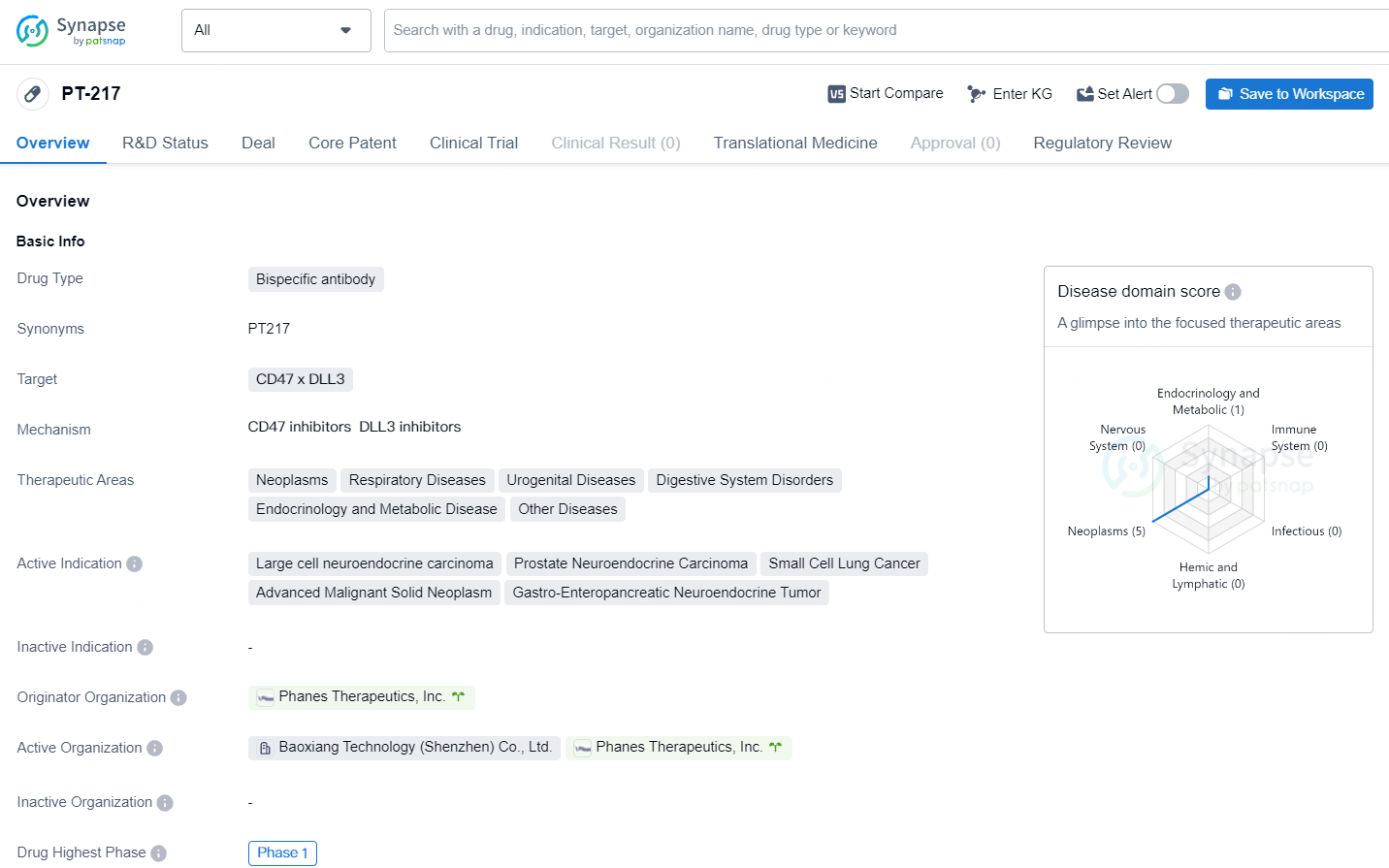
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
In 2022, the FDA awarded PT217 the orphan drug status for treating SCLC, and it also received the Fast Track designation for its use in extensive-stage small cell lung cancer patients who have shown disease progression after treatment with platinum-based chemotherapy, with or without the inclusion of a checkpoint inhibitor.
Phanes is actively recruiting participants for a multi-center Phase I study on PT217. This trial, referred to as the SKYBRIDGE study, aims to assess PT217's safety, tolerability, pharmacodynamics, and initial effectiveness in individuals with advanced or therapy-resistant cancers that show DLL3 expression.
Phanes' upcoming research phase will explore the efficacy of PT217 as a part of combination therapy for SCLC, LCNEC, and EP-NECs. This stage involves a clinical partnership with Roche to test PT217 together with atezolizumab for treating these conditions.
Rita Laeufle, MD, PhD, Chief Medical Officer at Phanes, expressed enthusiasm about the collaboration with Roche: “Partnering with Roche represents an innovative step forward in treating cancers such as SCLC, LCNEC, and EP-NECs, all of which commonly express DLL3, a critical therapeutic target. The intersecting mechanisms of PT217 and atezolizumab may enhance patient outcomes. This partnership is a significant achievement in Phanes’ mission to pioneer novel cancer therapies.”
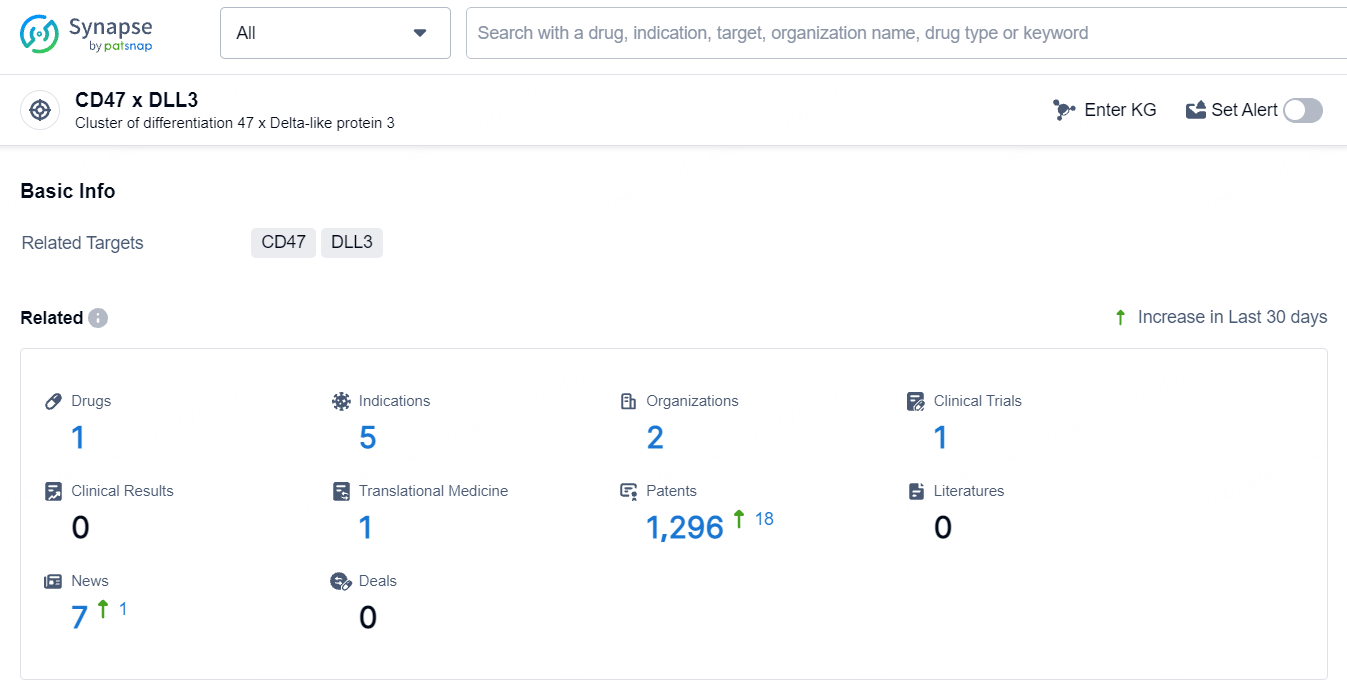
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 11, 2024, there are 1 investigational drugs for the CD47 and DLL3 targets, including 5 indications, 2 R&D institutions involved, with related clinical trials reaching 1, and as many as 1296 patents.
PT-217 has been granted Fast Track status and Orphan Drug designation. Fast Track status is given to drugs that show potential in addressing unmet medical needs, allowing for expedited development and review processes. Orphan Drug designation is granted to drugs that target rare diseases, providing incentives for their development.