PharmAbcine Begins Early-Stage PMC-309/KEYTRUDA Trial for Severe Solid Cancers
PharmAbcine Inc., an enterprise at the clinical developmental stage dedicated to pioneering antibody treatments, has declared the commencement of a Phase 1a/b study with the initiation of administering PMC-309 to individuals diagnosed with advanced or widespread solid neoplasms.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
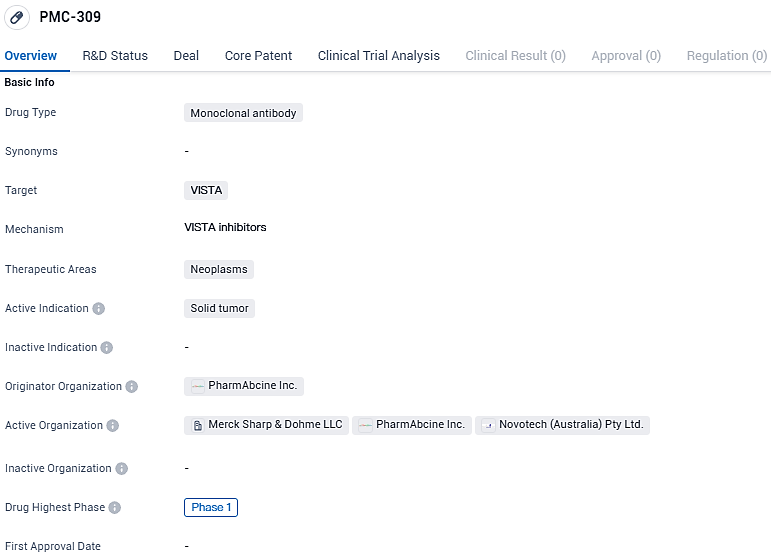
The therapeutic agent PMC-309, a selective IgG1 monoclonal antibody, targets VISTA on cells that suppress immune function and demonstrates robust affinity for this molecule under the varying pH levels encountered in the tumor microenvironment. PMC-309 works by disrupting VISTA, adopting a distinct mode of action that leads to the encouragement of T cell activity, stimulation of monocytes, and enhanced growth of M1 macrophages, all of which contribute to its potential to combat cancer.
The planned clinical research involving PMC-309 is split into two stages: an initial Phase 1a followed by Phase 1b. The first phase will explore the solo application of PMC-309 as well as its use in conjunction with KEYTRUDA® (pembrolizumab), with an eye on identifying the highest dose that patients can tolerate and establishing the dose suggested for Phase 2 studies.
During the subsequent Phase 1b segment, the research will focus on assessing the safety and acceptability of both the solitary use of PMC-309 and its combined application with KEYTRUDA® at the determined recommended Phase 2 dose (RP2D). The investigation is set to be carried out across four research centers in Australia, aiming to involve a cohort of 67 individuals.
Dr. Jin-San Yoo, who holds the positions of President and CEO of PharmAbcine, expressed the following: "Our ongoing clinical investigation is directed toward evaluating the safety of PMC-309 in humans and investigating the therapeutic viability of using PMC-309 both alone and in combo with KEYTRUDA®. Our commitment is steadfast in offering innovative treatment avenues for cancer sufferers who are currently faced with limited options, underlining our resolve to push forward the progress of cancer therapies."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
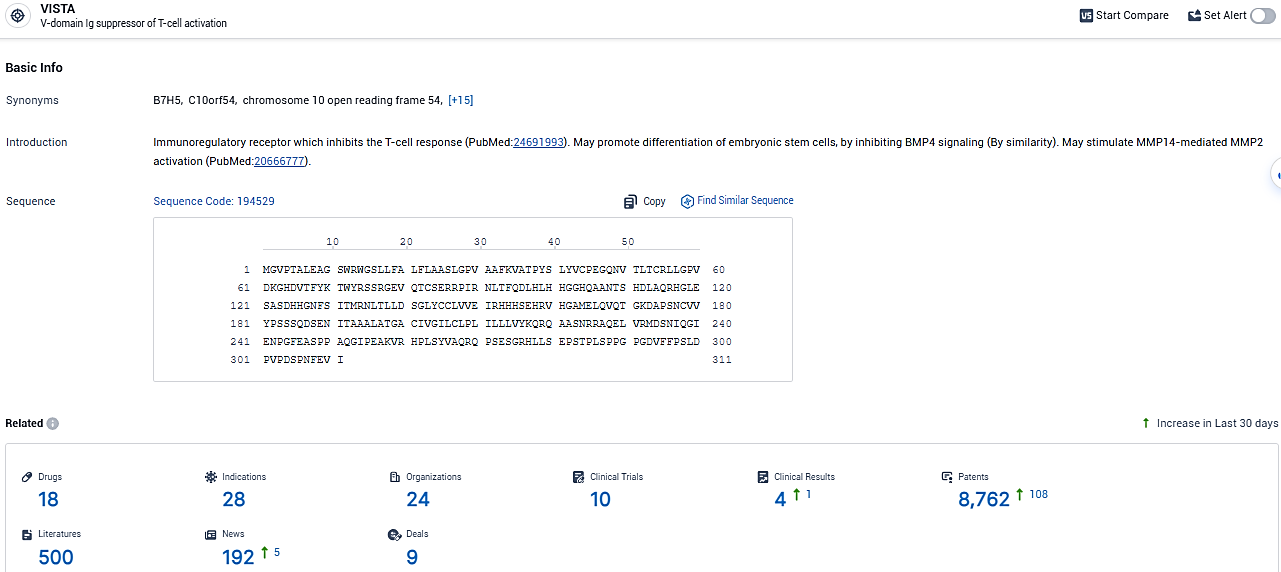
According to the data provided by the Synapse Database, As of January 29, 2024, there are 18 investigational drugs for the VISTA target, including 28 indications, 24 R&D institutions involved, with related clinical trials reaching 10, and as many as 8762 patents.
PMC-309 targets VISTA and is being developed for the treatment of neoplasms, specifically solid tumors. The drug has reached Phase 1 of clinical development, indicating progress in its evaluation for safety and efficacy. Further research and clinical trials will be necessary to determine the potential of PMC-309 as a therapeutic option for patients with solid tumors.