Pharmaceutical Insights: Exemestane's R&D Progress and its Mechanism of Action on Drug Target
Exemestane's R&D Progress
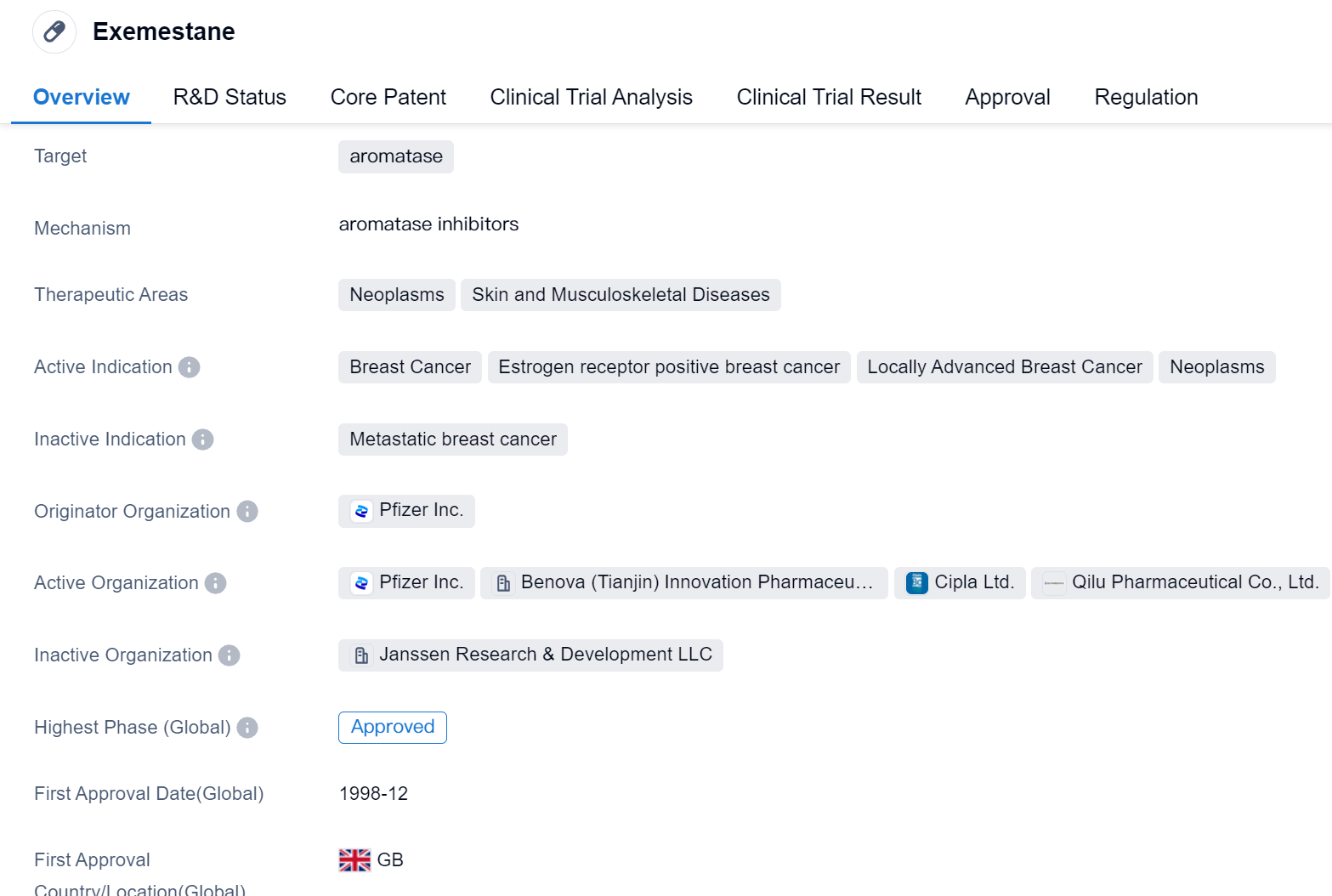
Exemestane is a small molecule drug that targets aromatase, an enzyme involved in the production of estrogen. It is primarily used in the treatment of various neoplasms, skin, and musculoskeletal diseases. The drug has shown efficacy in treating breast cancer, particularly estrogen receptor-positive breast cancer and locally advanced breast cancer.
Exemestane was first approved for use in the United Kingdom in December 1998. It is developed and marketed by Pfizer Inc., a renowned pharmaceutical company. The drug has received approval for use in multiple countries, including China, indicating its global recognition and acceptance.
The approval of Exemestane as an orphan drug suggests that it is intended to treat rare diseases or conditions that affect a small number of patients. This designation often provides certain incentives to the manufacturer, such as market exclusivity and financial benefits, to encourage the development of drugs for rare diseases.
As a small molecule drug, Exemestane is chemically synthesized and typically administered orally. It works by inhibiting the activity of aromatase, thereby reducing the production of estrogen. This is particularly important in the treatment of estrogen receptor-positive breast cancer, where estrogen plays a significant role in tumor growth.
The drug has undergone rigorous clinical trials to establish its safety and efficacy. Its highest phase of development, both globally and in China, is approved, indicating that it has successfully met the necessary regulatory requirements in these regions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Exemestane: aromatase inhibitors
Aromatase inhibitors are a type of medication used in the treatment of hormone receptor-positive breast cancer. These inhibitors work by blocking the activity of an enzyme called aromatase, which is responsible for the production of estrogen in postmenopausal women. By reducing estrogen levels, aromatase inhibitors help to slow down or stop the growth of breast cancer cells that rely on estrogen for their growth. This type of medication is typically prescribed to women who have gone through menopause and have hormone receptor-positive breast cancer. Aromatase inhibitors are often used as part of adjuvant therapy or as a treatment option for advanced or metastatic breast cancer.
Drug Target R&D Trends for Exemestane
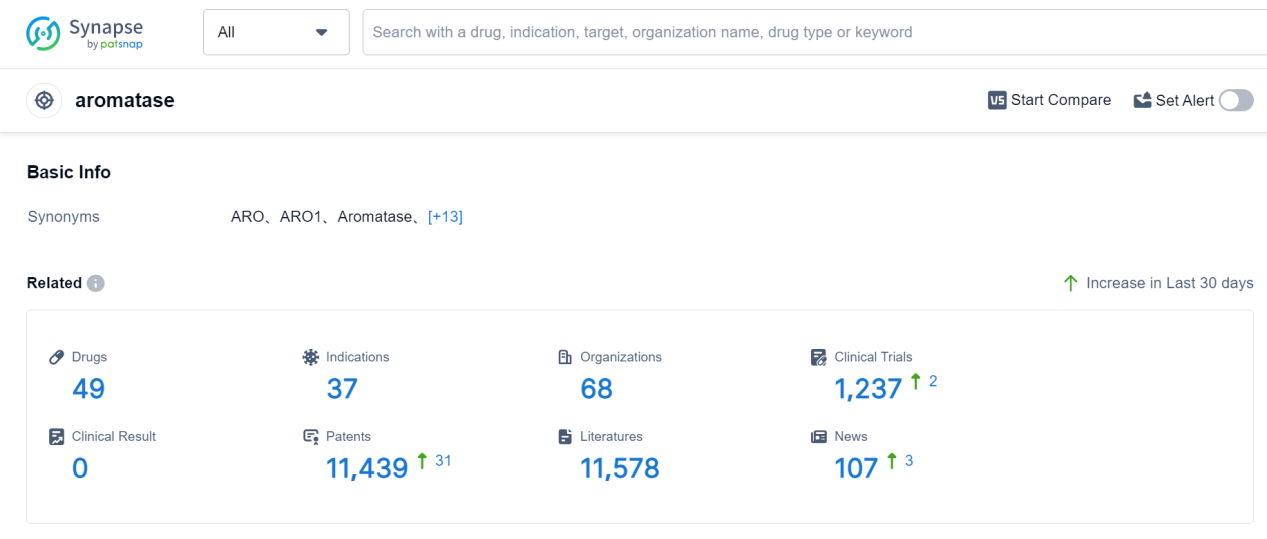
Aromatase is an enzyme that plays a crucial role in the human body by converting androgens (male hormones) into estrogens (female hormones). It is primarily found in various tissues, including the ovaries, testes, adrenal glands, and fat cells. Aromatase is responsible for the synthesis of estrogen, which is essential for the development and functioning of reproductive organs, bone health, and regulation of the menstrual cycle. Additionally, aromatase is involved in the production of estrogen in postmenopausal women, as it becomes the primary source of estrogen after the ovaries stop producing it. Understanding the role of aromatase is vital in the development of therapies targeting hormone-related conditions such as breast cancer and hormonal imbalances.
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 49 aromatase drugs worldwide, from 68 organizations, covering 37 indications, and conducting 1237 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Exemestane is a small molecule drug developed by Pfizer Inc. that targets aromatase. It is primarily used in the treatment of neoplasms, skin, and musculoskeletal diseases, with a specific focus on breast cancer, including estrogen receptor-positive and locally advanced breast cancer. The drug has received approval in multiple countries, including the United Kingdom and China, and is designated as an orphan drug. Its approval and successful development highlight its potential as an effective treatment option in the field of biomedicine.