Phase III Trials Show Genentech's Gazyva Surpasses Standard Lupus Nephritis Treatments
Genentech, part of the Roche Group, revealed that the Phase III REGENCY study of Gazyva® (obinutuzumab) in patients with active lupus nephritis yielded positive topline results. In the trial, a higher percentage of participants receiving Gazyva in combination with standard treatment (mycophenolate mofetil and glucocorticoids) reached a complete renal response (CRR) at 76 weeks compared to those who received only the standard treatment. The safety profile observed was consistent with Gazyva's established safety characteristics, and no new safety concerns were reported.
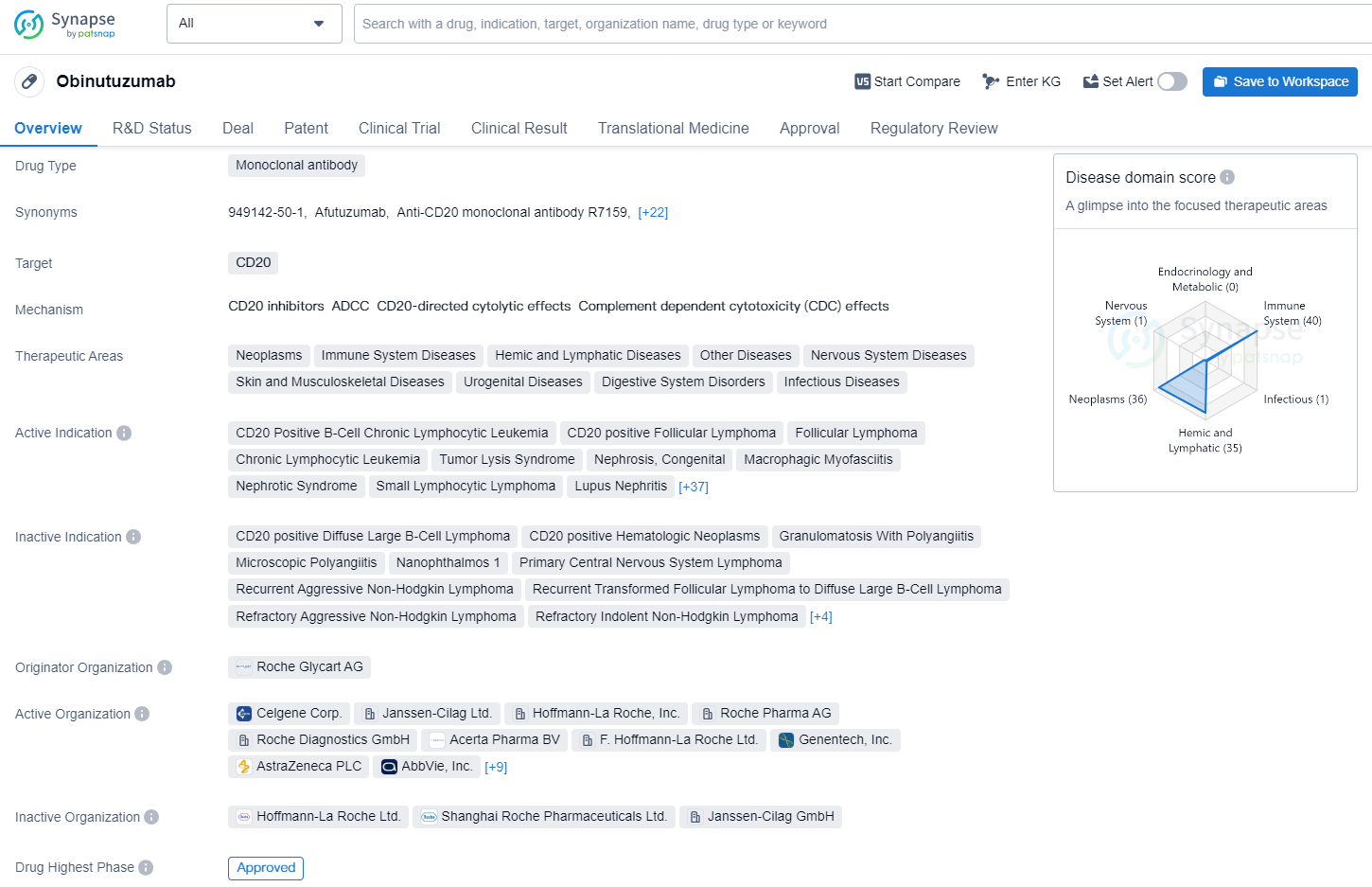
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"Gazyva demonstrated a strong complete renal response rate in lupus nephritis, which correlates with prolonged kidney function preservation and postponement or prevention of end-stage kidney disease," stated Levi Garraway, M.D., Ph.D., chief medical officer, and head of Global Product Development. "Given that patients with advanced kidney disease often require dialysis or transplants, these results could signify a significant advancement for individuals battling this debilitating condition.”
“The announcement that the Phase III REGENCY study has achieved its primary endpoint is highly encouraging,” remarked Dr. Brad H. Rovin, Director of Nephrology and Medical Director of the Center for Clinical Research Management at The Ohio State University Wexner Medical Center, and principal investigator for the REGENCY study. "The REGENCY results are compelling. Obinutuzumab presents a promising new treatment option for the lupus community to manage this challenging disease associated with substantial morbidity."
Two crucial secondary endpoints indicated statistically significant and clinically meaningful benefits with Gazyva-namely, the proportion of patients reaching CRR with reduced corticosteroid use, and an enhanced proteinuric response (both at 76 weeks). These endpoints are essential markers for better disease control in lupus nephritis. Other secondary endpoints did not achieve statistical significance, but Gazyva showed numerically higher responses in several endpoints.
Data are being communicated to health regulatory authorities, including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency, aiming to make this potential new lupus nephritis treatment available promptly. Data are also being prepared for publication in a medical journal and for presentation at an upcoming medical conference.
Lupus nephritis is a potentially fatal manifestation of an autoimmune disorder affecting about 1.7 million people globally, mostly women and predominantly women of color and of childbearing age. In lupus nephritis, pathogenic B cells drive ongoing inflammation resulting in kidney damage. Despite available treatments, up to one-third of individuals will progress to end-stage kidney disease within a decade, with dialysis or transplant as the only interventions and a high mortality risk. Evidence suggests that Gazyva depletes pathogenic B cells, aiding in minimizing additional kidney damage and potentially averting or delaying the progression to end-stage kidney disease.
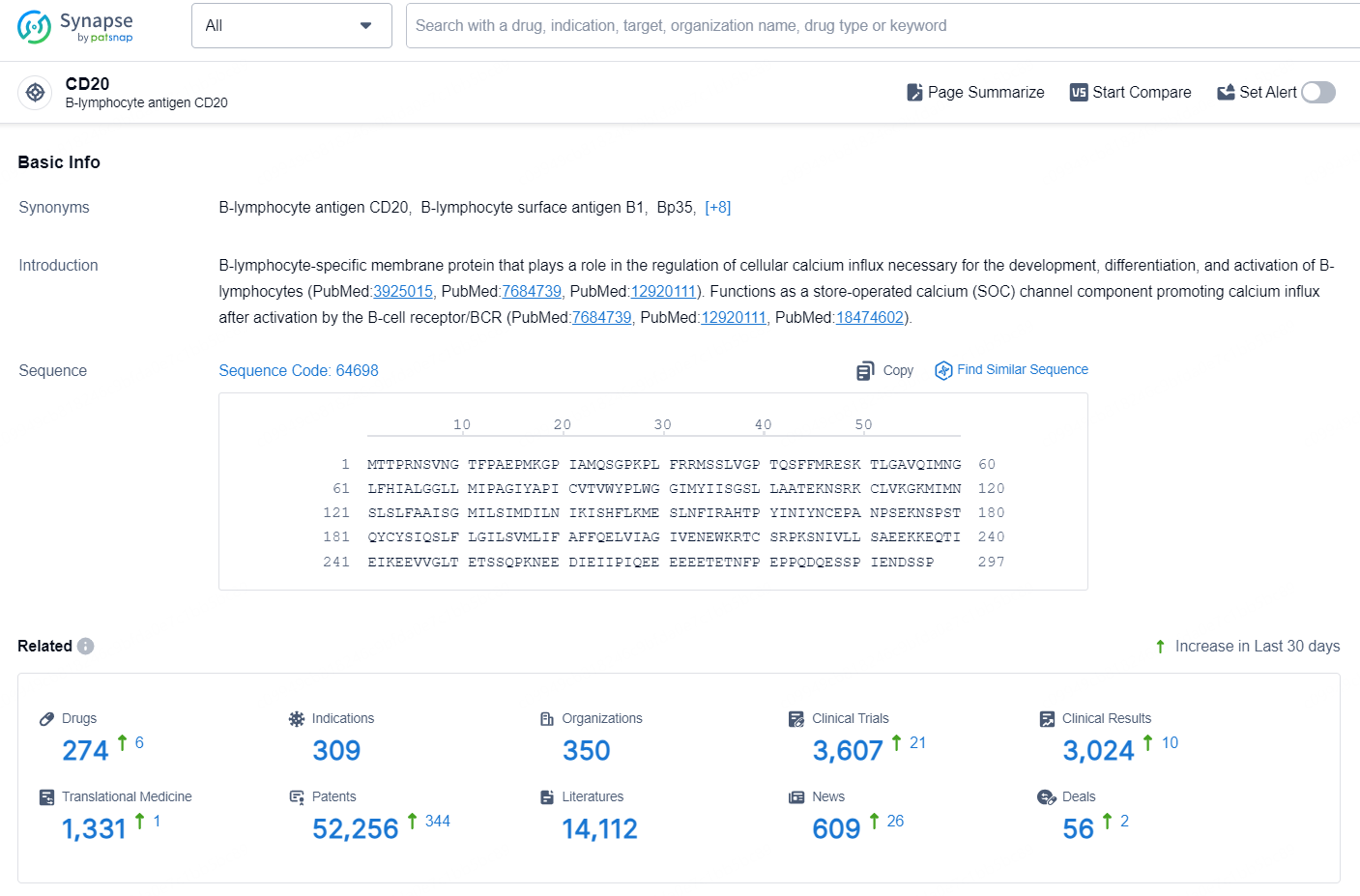
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of September 29, 2024, there are 274 investigational drugs for the CD20 target, including 309 indications, 350 R&D institutions involved, with related clinical trials reaching 3607, and as many as 52256 patents.
Obinutuzumab is a monoclonal antibody drug developed by Roche Glycart AG, and it was first approved for use in the United States in November 2013. The drug targets CD20, making it effective in treating a wide range of therapeutic areas, including neoplasms, immune system diseases, hemic and lymphatic diseases, nervous system diseases, and others. The drug has received approvals for various indications, including CD20-positive B-cell chronic lymphocytic leukemia, follicular lymphoma, tumor lysis syndrome, systemic lupus erythematosus, and metastatic colorectal carcinoma, among others.