Pheast Releases Initial Preclinical Findings on Anti-CD24 Macrophage Checkpoint Inhibitor, PHST001
Pheast Therapeutics, a biotechnology firm focused on pioneering macrophage checkpoint therapies to combat cancer, revealed the initial showcasing of preclinical data for PHST001, an anti-CD24 antibody drug candidate specifically developed to inhibit a critical macrophage "don't eat me" signal present on cancer cells. The data, to be presented at the 20th Annual PEGS Boston Summit, demonstrate that PHST001 can effectively trigger an anti-cancer immune response and enhance therapeutic outcomes in in vivo models.
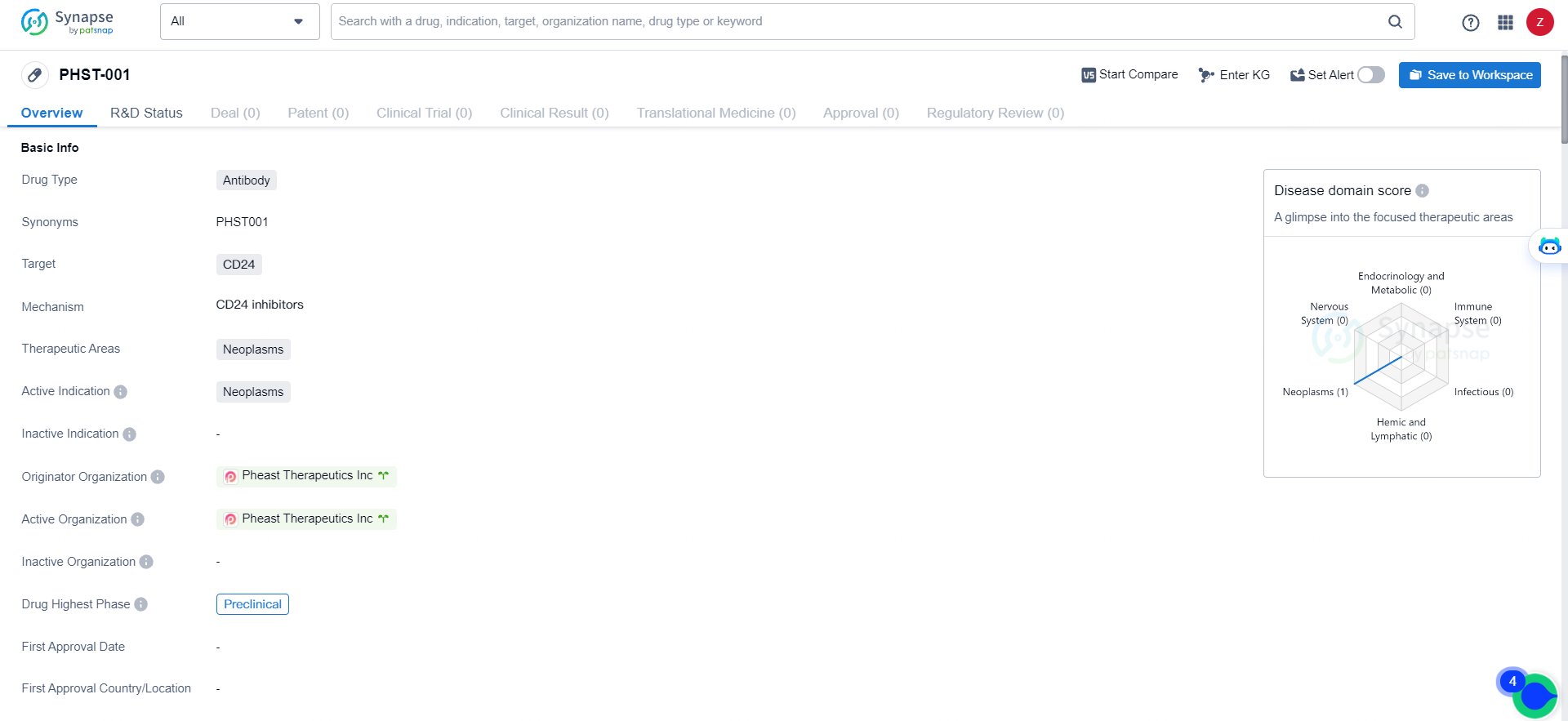
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"Pheast’s leading drug candidate, PHST001, is an exceptionally sophisticated and well-expressed antibody targeting the novel identifier, CD24," stated Roy Maute, Ph.D., Cofounder and CEO of Pheast Therapeutics. "Our preclinical trials have exhibited significant effectiveness in stringent mouse model systems, suggesting potential clinical success in malignancies where existing immunotherapies have shown limited results and high unmet medical needs persist. The Pheast team is swiftly advancing to support the submission of an investigational new drug application, aiming to commence clinical trials in patients within the first half of the upcoming year."
CD24 is identified as a newly discovered immune checkpoint that many human cancers, such as ovarian and triple negative breast cancer, heavily express. High levels of CD24 expression are associated with poor prognosis across various cancer types. CD24 engages with the macrophage receptor Siglec-10, thereby protecting cancer cells from macrophage attacks. Pheast has developed PHST001 to bind both recombinant CD24 and cell surface CD24 with superior affinity and specificity, inhibiting Siglec-10 interaction.
The scientists at Pheast have proven that inhibiting CD24 with PHST001 facilitates macrophage phagocytosis of several cancer types in vitro. Furthermore, they have demonstrated substantial efficacy of PHST001 in vivo, with some preclinical models showing monotherapy with PHST001 leads to complete tumor regression in all treated mice.
"These findings highlight Pheast’s robust internal capabilities in antibody discovery and protein engineering, enabling quick identification and development of therapeutic monoclonal antibodies," noted John Burg, Ph.D., Senior Director of Protein Sciences. "Our pioneering platform for the identification of tumor-specific immune checkpoints tailored for precision immuno-oncology therapies will facilitate the exploration of additional targets and the advancement of new pipeline programs."
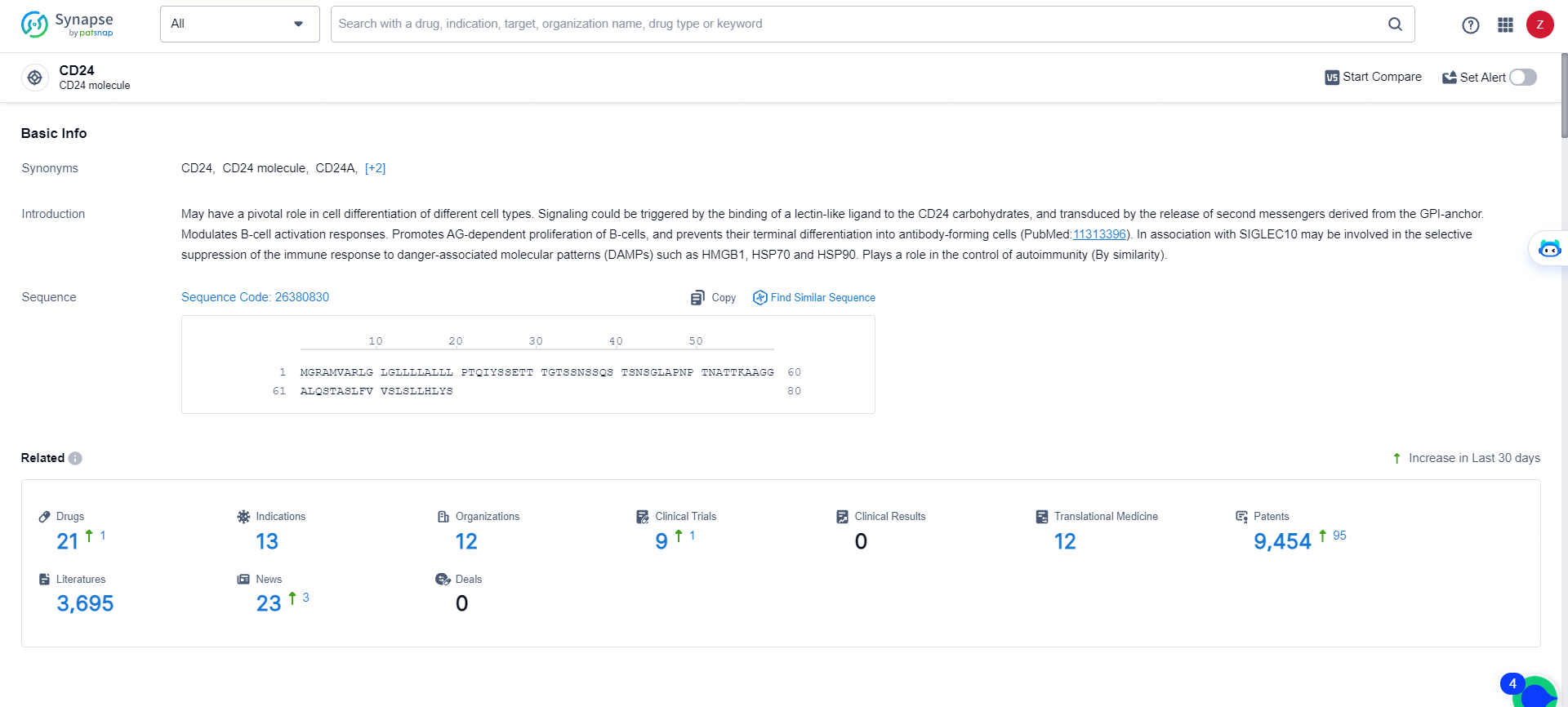
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of May 16, 2024, there are 21 investigational drugs for the CD24 targets, including 13 indications, 12 R&D institutions involved, with related clinical trials reaching 9, and as many as 9454 patents.
PHST-001 suggests that it is a novel antibody drug with a specific focus on neoplasms, particularly in targeting CD24. Its current status in the preclinical phase indicates that it is still in the early stages of development, and further research and clinical trials will be needed to determine its potential as a treatment for cancer. As such, [PHST-001] represents an interesting prospect in the field of biomedicine and pharmaceutical development.